Label: FELODIPINE tablet, film coated, extended release
- NDC Code(s): 68462-233-01, 68462-233-10, 68462-233-11, 68462-233-90, view more
- Packager: Glenmark Pharmaceuticals Inc., USA
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 11, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Rx Only
-
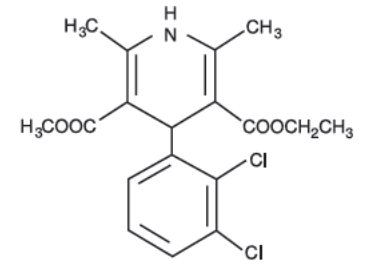
DESCRIPTIONFelodipine extended-release tablets USP are a calcium antagonist (calcium channel blocker). Felodipine is a dihydropyridine derivative that is chemically described as ± ethyl methyl ...
-
CLINICAL PHARMACOLOGYMechanism of Action - Felodipine is a member of the dihydropyridine class of calcium channel antagonists (calcium channel blockers). It reversibly competes with nitrendipine and/or other calcium ...
-
INDICATIONS AND USAGEFelodipine tablets are indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure lowers the risk of fatal and non-fatal cardiovascular events, primarily strokes ...
-
CONTRAINDICATIONSFelodipine is contraindicated in patients who are hypersensitive to this product.
-
PRECAUTIONSGeneral - Hypotension - Felodipine, like other calcium antagonists, may occasionally precipitate significant hypotension and, rarely, syncope. It may lead to reflex tachycardia which in ...
-
ADVERSE REACTIONS In controlled studies in the United States and overseas, approximately 3000 patients were treated with felodipine as either the extended-release or the immediate-release formulation. The most ...
-
OVERDOSAGEOral doses of 240 mg/kg and 264 mg/kg in male and female mice, respectively, and 2390 mg/kg and 2250 mg/kg in male and female rats, respectively, caused significant lethality. In a suicide ...
-
DOSAGE AND ADMINISTRATION The recommended starting dose is 5 mg once a day. Depending on the patient's response, the dosage can be decreased to 2.5 mg or increased to 10 mg once a day. These adjustments should occur ...
-
HOW SUPPLIEDFelodipine extended-release tablets USP, 2.5 mg are green colored film coated, circular shaped, biconvex tablets engraved with “G19” on one side of the tablet and plain on the other side. They are ...
-
SPL UNCLASSIFIED SECTIONManufactured by: Glenmark Pharmaceuticals Ltd. Colvale-Bardez, Goa 403 513, India - Manufactured for: Glenmark Pharmaceuticals Inc., USA - Mahwah, NJ 07430 - Questions? 1 ...
-
Package/Label Display PanelNDC 68462-233-01 - Felodipine Extended-Release Tablets USP - 2.5 mg
-
Package/Label Display PanelNDC 68462-234-01 - Felodipine Extended-Release Tablets USP - 5 mg
-
Package/Label Display PanelNDC 68462-235-01 - Felodipine Extended-Release Tablets USP - 10 mg
-
INGREDIENTS AND APPEARANCEProduct Information