Label: METRONIDAZOLE gel
- NDC Code(s): 68462-184-49
- Packager: GLENMARK PHARMACEUTICALS INC., USA
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 27, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONFOR INTRAVAGINAL USE ONLY - NOT FOR OPHTHALMIC, DERMAL, OR ORAL USE
-
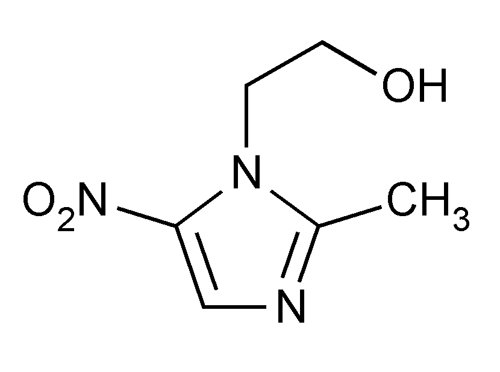
DESCRIPTIONMetronidazole Vaginal Gel is the intravaginal dosage form of the synthetic antibacterial agent, metronidazole, USP at a concentration of 0.75%. Metronidazole is a member of the imidazole class of ...
-
CLINICAL PHARMACOLOGY Normal Subjects - Following a single, intravaginal 5 g dose of metronidazole vaginal gel (equivalent to 37.5 mg of metronidazole) to 12 normal subjects, a mean maximum serum metronidazole ...
-
INDICATIONS AND USAGE Metronidazole vaginal gel is indicated in the treatment of bacterial vaginosis (formerly referred to as Haemophilus vaginitis, Gardnerella vaginitis, nonspecific vaginitis, Corynebacterium ...
-
CONTRAINDICATIONS Metronidazole vaginal gel is contraindicated in patients with a prior history of hypersensitivity to metronidazole, parabens, other ingredients of the formulation, or other nitroimidazole ...
-
WARNINGS Convulsive Seizures and Peripheral Neuropathy - Convulsive seizures and peripheral neuropathy, the latter characterized mainly by numbness or paresthesia of an extremity, have been reported in ...
-
PRECAUTIONS Metronidazole vaginal gel affords minimal peak serum levels and systemic exposure (AUCs) of metronidazole compared to 500 mg oral metronidazole dosing. Although these lower levels of exposure are ...
-
ADVERSE EVENTS Clinical Trials - There were no deaths or serious adverse events related to drug therapy in clinical trials involving 800 non-pregnant women who received metronidazole vaginal gel. In a ...
-
OVERDOSAGE There is no human experience with overdosage of metronidazole vaginal gel. Vaginally applied metronidazole gel, 0.75% could be absorbed in sufficient amounts to produce systemic effects (see ...
-
DOSAGE AND ADMINISTRATION The recommended dose is one applicator full of metronidazole vaginal gel (approximately 5 grams containing approximately 37.5 mg of metronidazole) intravaginally once or twice a day for 5 days ...
-
HOW SUPPLIED Metronidazole Vaginal Gel, 0.75% is supplied in a 70 g tube and packaged with 5 vaginal applicators. The NDC number for the 70 g tube is 68462-184-49. Store at controlled room temperature 15°C to ...
-
Clinical Studies In a randomized, single-blind clinical trial of non-pregnant women with bacterial vaginosis who received metronidazole vaginal gel daily for 5 days, the clinical cure rates for evaluable patients ...
-
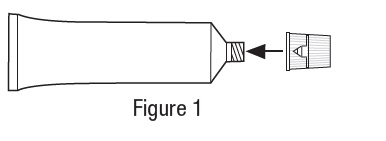
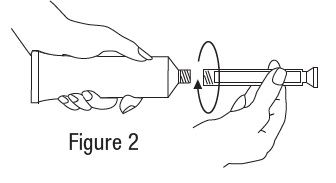
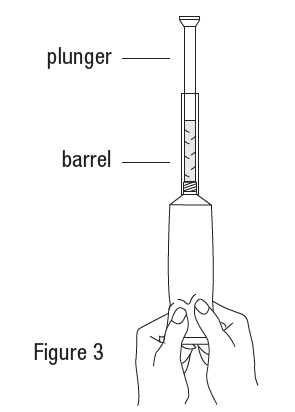
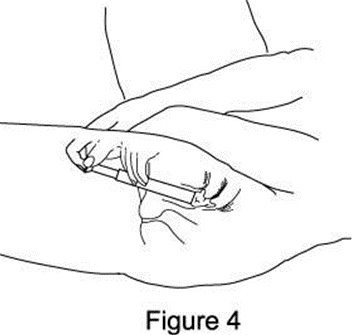
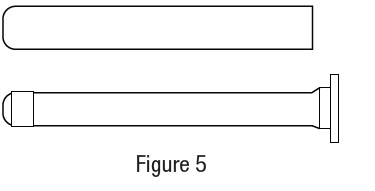
Metronidazole Vaginal Gel, 0.75% DIRECTIONS FOR USE - 1. Filling the applicator - • Remove cap and puncture metal seal on tube with the pointed tip of cap (see Figure 1). • Screw end of applicator onto tube (see Figure ...
-
PRINCIPAL DISPLAY PANEL

-
INGREDIENTS AND APPEARANCEProduct Information