Label: ZOLMITRIPTAN tablet, orally disintegrating
- NDC Code(s): 68382-715-01, 68382-715-06, 68382-715-10, 68382-715-16, view more
- Packager: Zydus Pharmaceuticals USA Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 8, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ZOLMITRIPTAN ORALLY DISINTEGRATING TABLETS safely and effectively. See full prescribing information for ZOLMITRIPTAN ORALLY ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEZolmitriptan orally disintegrating tablets are indicated for the acute treatment of migraine with or without aura in adults. Limitations of Use - Only use zolmitriptan if a clear diagnosis ...
-
2 DOSAGE AND ADMINISTRATION2.1 Dosing Information - The recommended starting dose of zolmitriptan tablets is 1.25 mg or 2.5 mg. The 1.25 mg dose can be achieved by manually breaking the functionally-scored 2.5 mg tablet ...
-
3 DOSAGE FORMS AND STRENGTHS2.5 mg tablets are white/mottled white to cream white, round, flat-faced uncoated tablet, debossed with '715' on one side and plain on other the side. 5 mg tablets are white/mottled white to ...
-
4 CONTRAINDICATIONSZolmitriptan orally disintegrating tablets are contraindicated in patients with: Ischemic coronary artery disease (angina pectoris, history of myocardial infarction, or documented silent ...
-
5 WARNINGS AND PRECAUTIONS5.1 Myocardial Ischemia, Myocardial Infarction, and Prinzmetal Angina - Zolmitriptan is contraindicated in patients with ischemic or vasospastic coronary artery disease (CAD). There have been ...
-
6 ADVERSE REACTIONSThe following adverse reactions are described elsewhere in other sections of the prescribing information: Myocardial Ischemia, Myocardial Infarction, and Prinzmetal Angina [see Warnings and ...
-
7 DRUG INTERACTIONS7.1 Ergot-containing Drugs - Ergot-containing drugs have been reported to cause prolonged vasospastic reactions. Because these effects may be additive, use of ergotamine containing or ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no adequate data on the developmental risk associated with the use of zolmitriptan in pregnant women. In reproductive toxicity studies in rats and ...
-
10 OVERDOSAGEThere is no experience with acute overdose of zolmitriptan. Clinical study subjects who received single 50 mg oral doses of zolmitriptan commonly experienced sedation. There is no specific ...
-
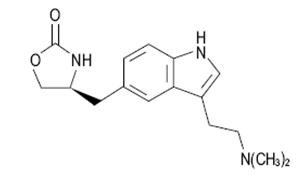
11 DESCRIPTIONZolmitriptan orally disintegrating tablets contain zolmitriptan, which is a selective 5-hydroxytryptamine1B/1D (5 -HT1B/1D) receptor agonist. Zolmitriptan is chemically designated as ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Zolmitriptan binds with high affinity to human recombinant 5-HT1D and 5-HT1B receptors, and moderate affinity for 5-HT1A receptors. The N-desmethyl metabolite also has ...
-
13 NONCLINICAL TOXICOLOGY13.3 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Zolmitriptan was administered to mice and rats at doses up to 400 mg/kg/day. Mice were dosed for 85 weeks (males) and ...
-
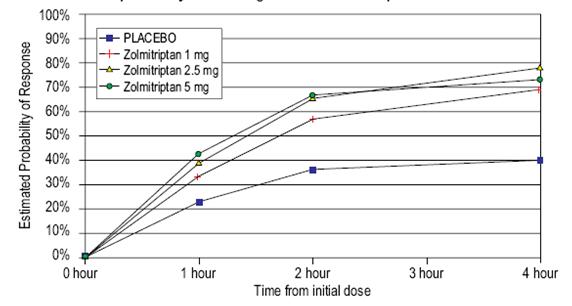
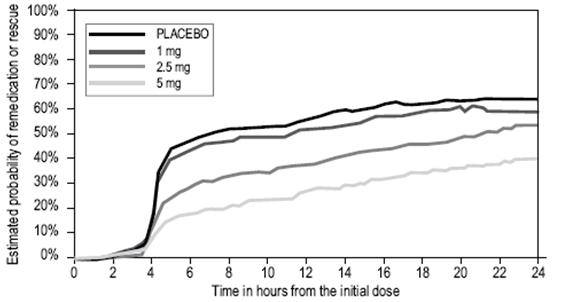
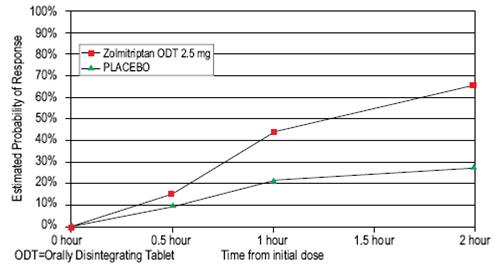
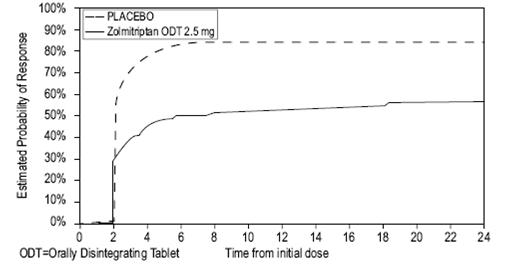
14 CLINICAL STUDIESZolmitriptan Tablets - The efficacy of zolmitriptan tablets in the acute treatment of migraine headaches was demonstrated in five randomized, double-blind, placebo-controlled studies (Studies 1 ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGZolmitriptan Orally Disintegrating Tablets USP, 2.5 mg are white/mottled white to cream white, round, flat-faced, uncoated tablet, debossed with '715' on one side and plain on the other side and ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Myocardial Ischemia and/or Infarction, Prinzmetal's Angina, Other Vasospastic Reactions, and Cerebrovascular ...
-
SPL UNCLASSIFIED SECTIONManufactured by: Zydus Lifesciences Ltd. Ahmedabad, India - Distributed by: Zydus Pharmaceuticals (USA) Inc. Pennington, NJ 08534 - Rev.: 01/23
-
PATIENT PACKAGE INSERTPatient Information - Zolmitriptan (zole'' mi trip' tan) Orally Disintegrating Tablets, USP - Please read this information before you start taking zolmitriptan and each time you renew your ...
-
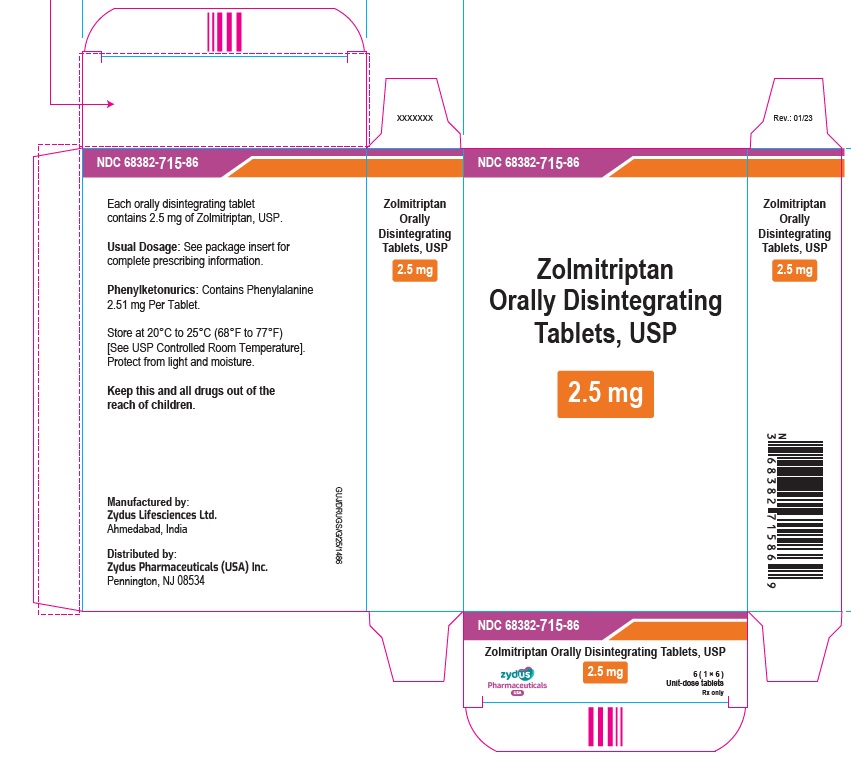
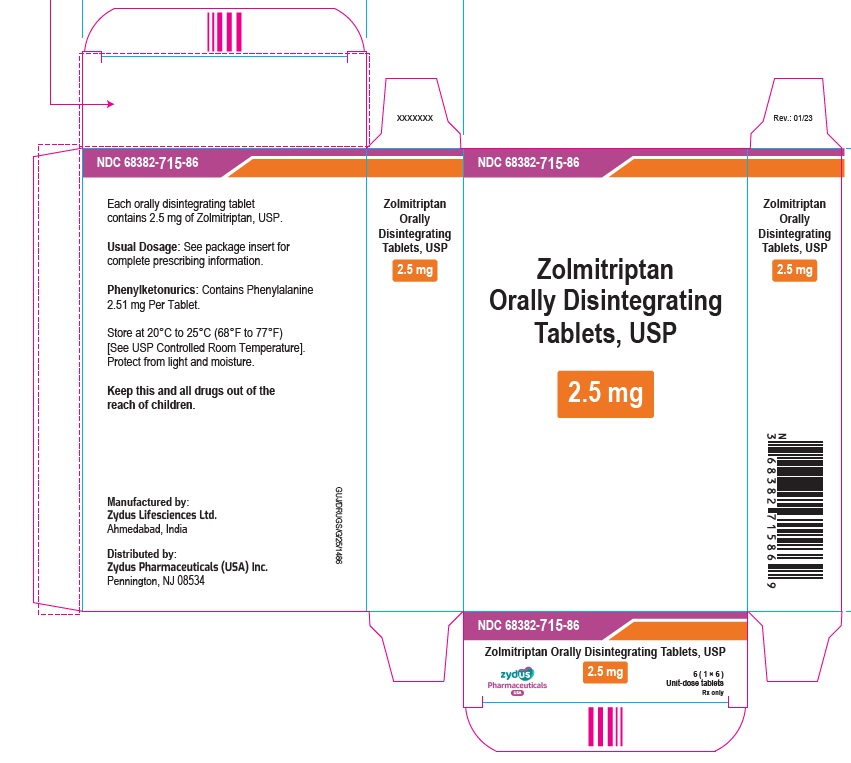
PRINCIPAL DISPLAY PANELNDC 68382-715-69 - Zolmitriptan Orally Disintegrating Tablets USP, 2.5 mg - Rx only - 6 Tablets - ZYDUS - NDC 68382-715-86 - Zolmitriptan Orally Disintegrating Tablets USP, 2.5 mg - Rx only - 6 (1×6 ...
-
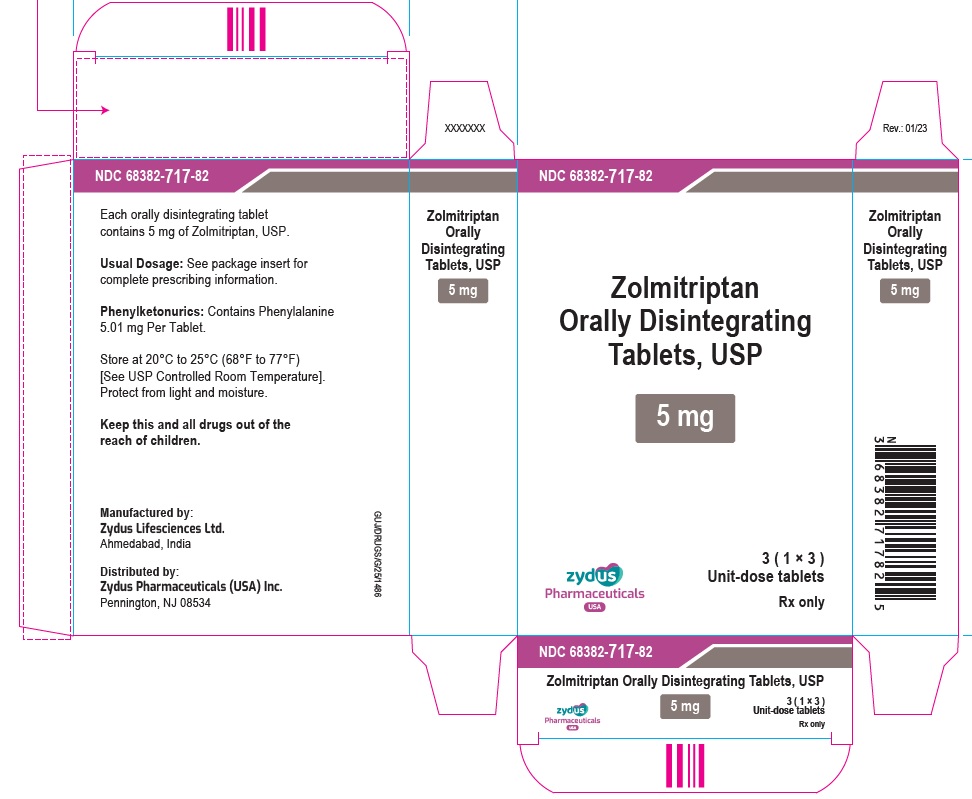
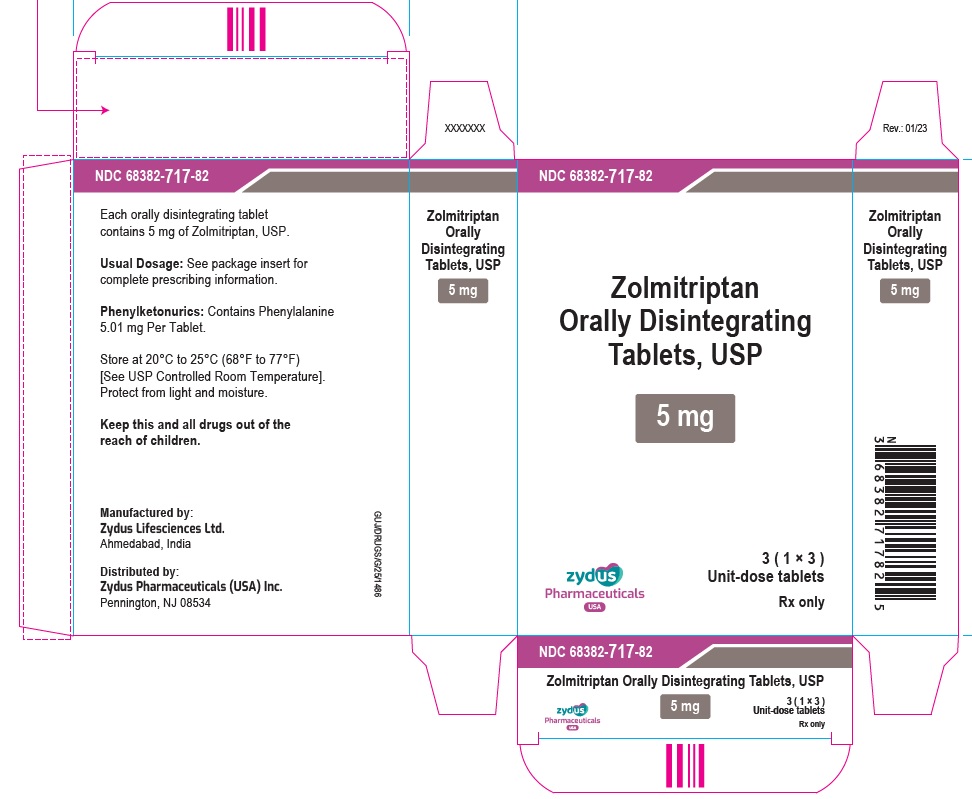
PRINCIPAL DISPLAY PANELNDC 68382-717-87 - Zolmitriptan Orally Disintegrating Tablets USP, 5 mg - Rx only - 3 Tablets - ZYDUS - NDC 68382-717-82 - Zolmitriptan Orally Disintegrating Tablets USP, 5 mg - Rx only - 3 (1×3) Unit-dose ...
-
INGREDIENTS AND APPEARANCEProduct Information