Label: ISOSORBIDE MONONITRATE tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 68382-650-01, 68382-650-05, 68382-651-01, 68382-651-05, view more - Packager: Zydus Pharmaceuticals (USA) Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 17, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx only
-
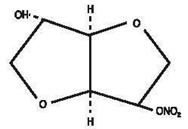
DESCRIPTIONIsosorbide Mononitrate (ISMN), an organic nitrate and the major biologically active metabolite of isosorbide dinitrate (ISDN), is a vasodilator with effects on both arteries and veins. Each ...
-
CLINICAL PHARMACOLOGYMechanism of Action - ISMN extended-release tablets are an oral extended-release formulation of ISMN, the major active metabolite of isosorbide dinitrate; most of the clinical activity of the ...
-
CLINICAL TRIALSControlled trials with Isosorbide mononitrate extended-release tablets have demonstrated antianginal activity following acute and chronic dosing. Administration of Isosorbide mononitrate ...
-
INDICATIONS AND USAGEIsosorbide mononitrate extended-release tablets are indicated for the prevention of angina pectoris due to coronary artery disease. The onset of action of oral ISMN is not sufficiently rapid for ...
-
CONTRAINDICATIONSIsosorbide mononitrate extended-release tablets are contraindicated in patients who have shown hypersensitivity or idiosyncratic reactions to other nitrates or nitrites.
-
WARNINGSAmplification of the vasodilatory effects of Isosorbide mononitrate extended-release tablets by sildenafil can result in severe hypotension. The time course and dose dependence of this ...
-
PRECAUTIONSGeneral - Severe hypotension, particularly with upright posture, may occur with even small doses of ISMN. This drug should therefore be used with caution in patients who may be volume depleted ...
-
DRUG & LABORATORY TEST INTERACTIONSNitrates and nitrites may interfere with the Zlatkis-Zak color reaction, causing falsely low readings in serum cholesterol determinations. Carcinogenesis, Mutagenesis, Impairment of ...
-
ADVERSE REACTIONSThe table below shows the frequencies of the adverse events that occurred in >5% of the subjects in three placebo-controlled North American studies, in which patients in the active treatment arm ...
-
OVERDOSAGEHemodynamic Effects - The ill effects of ISMN overdose are generally the results of ISMN's capacity to induce vasodilatation, venous pooling, reduced cardiac output, and hypotension. These ...
-
DOSAGE AND ADMINISTRATIONThe recommended starting dose of Isosorbide mononitrate extended-release tablets is 30 mg (given as a single 30 mg tablet or as 1/2 of a 60 mg tablet) or 60 mg (given as a single tablet) once ...
-
HOW SUPPLIEDIsosorbide mononitrate extended-release 30 mg tablets are oval, reddish pink, film-coated tablets, debossed "N" bisect "30" on one side and bisect on the other side, packaged as follows: NDC ...
-
Principal Display Panel - Bottle Label 30 mg 100 countZyGenerics - NDC 68382-650-01 - Isosorbide Mononitrate - Extended-Release Tablets - 30 mg - Rx only - 100 Tablets - P10232 - Rev: 02/15 - Each tablet contains: Isosorbide mononitrate ....30 ...
-
Principal Display Panel - Bottle Label 30 mg 500 countZyGenerics - NDC 68382-650-05 - Isosorbide Mononitrate - Extended-Release Tablets - 30 mg - Rx only - 500 Tablets - P10233 - Rev: 02/15 - Each tablet contains: Isosorbide mononitrate ....30 ...
-
Principal Display Panel - Bottle Label 60 mg 100 countZyGenerics - NDC 68382-651-01 - Isosorbide Mononitrate - Extended-Release Tablets - 60 mg - Rx only - 100 Tablets - P10234 - Rev: 02/15 - Each tablet contains: Isosorbide mononitrate ....60 ...
-
Principal Display Panel - Bottle Label 60 mg 500 countZyGenerics - NDC 68382-651-05 - Isosorbide Mononitrate - Extended-Release Tablets - 60 mg - Rx only - 500 Tablets - P10235 - Rev: 02/15 - Each tablet contains: Isosorbide mononitrate ....60 ...
-
Principal Display Panel - Bottle Label 120 mg 100 countZyGenerics - NDC 68382-652-01 - Isosorbide Mononitrate - Extended-Release Tablets - 120 mg - Rx only - 100 Tablets - P10236 - Rev: 02/15 - Each tablet contains: Isosorbide mononitrate ....120 ...
-
INGREDIENTS AND APPEARANCEProduct Information