Label: LIOTHYRONINE SODIUM tablet
- NDC Code(s): 68382-582-01, 68382-582-10, 68382-583-01, 68382-583-10, view more
- Packager: Zydus Pharmaceuticals (USA) Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated August 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use LIOTHYRONINE SODIUM TABLETS safely and effectively. See full prescribing information for LIOTHYRONINE SODIUM TABLETS. LIOTHYRONINE ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: NOT FOR TREATMENT OF OBESITY OR FOR WEIGHT LOSS
- Thyroid hormones, including liothyronine sodium, either alone or with other therapeutic agents, should not be used for the treatment of obesity or for weight loss.
- In euthyroid patients, doses within the range of daily hormonal requirements are ineffective for weight reduction.
- Larger doses may produce serious or even life-threatening manifestations of toxicity, particularly when given in association with sympathomimetic amines such as those used for their anorectic effects [see Adverse Reactions (6), Drug Interactions (7.7), and Overdosage (10)] .
-
1 INDICATIONS AND USAGE1.1 Hypothyroidism - Liothyronine sodium tablets are indicated as a replacement therapy in primary (thyroidal), secondary (pituitary), and tertiary (hypothalamic) congenital or acquired ...
-
2 DOSAGE AND ADMINISTRATION2.1 General Principles of Dosing - The dose of liothyronine sodium for hypothyroidism or pituitary Thyroid-Stimulating Hormone (TSH) suppression depends on a variety of factors including: the ...
-
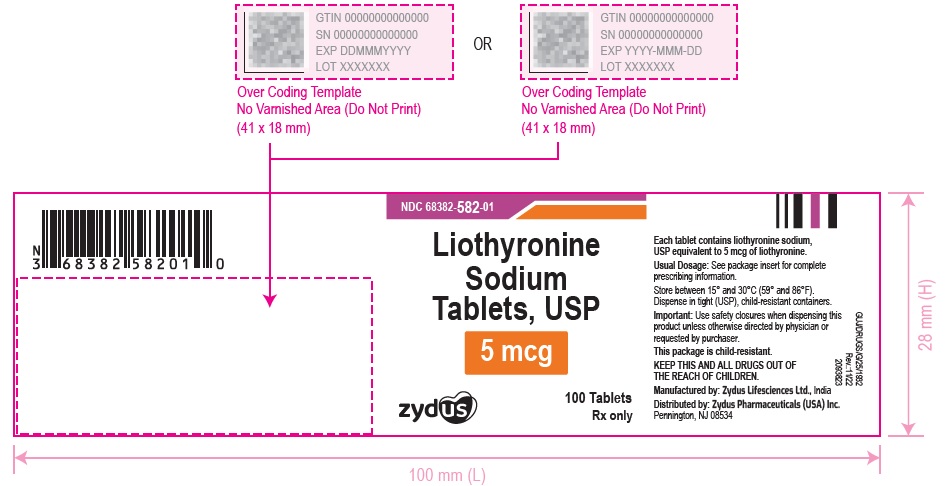
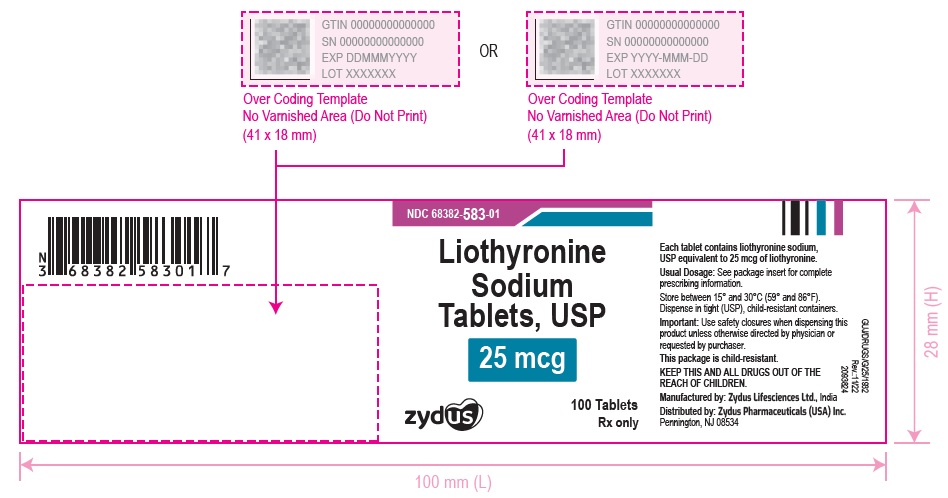
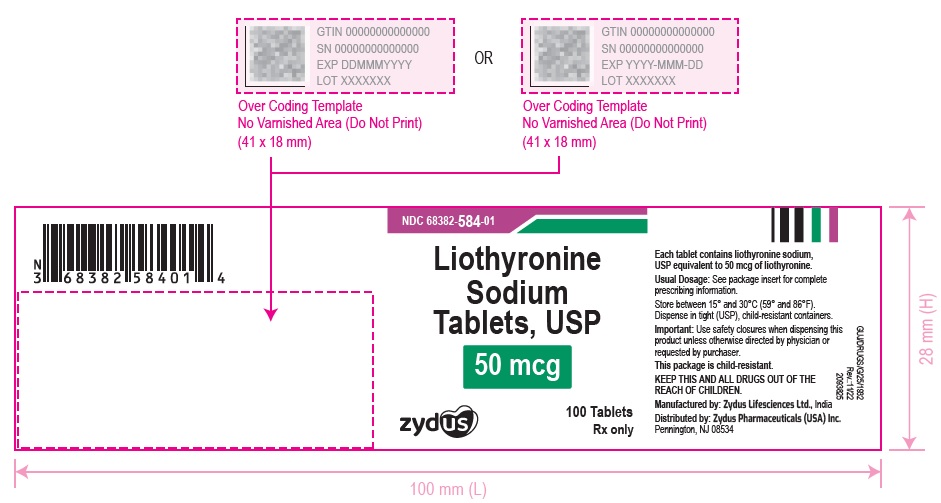
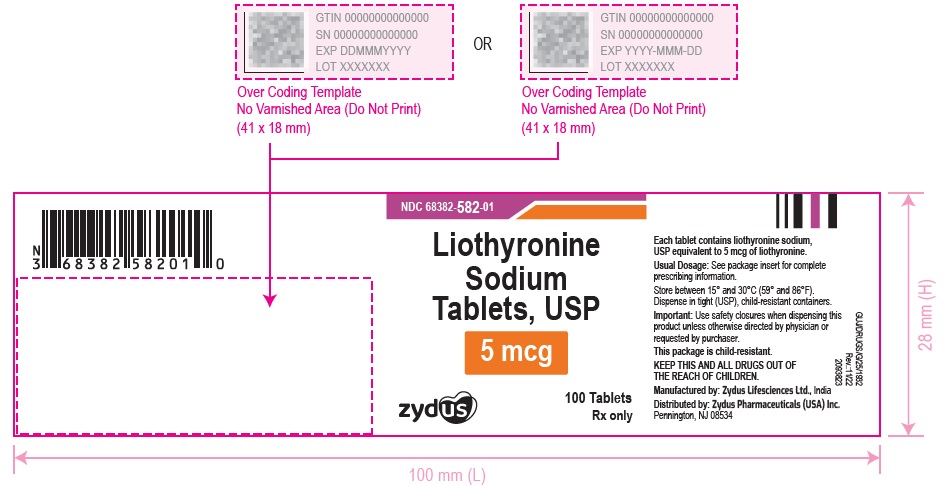
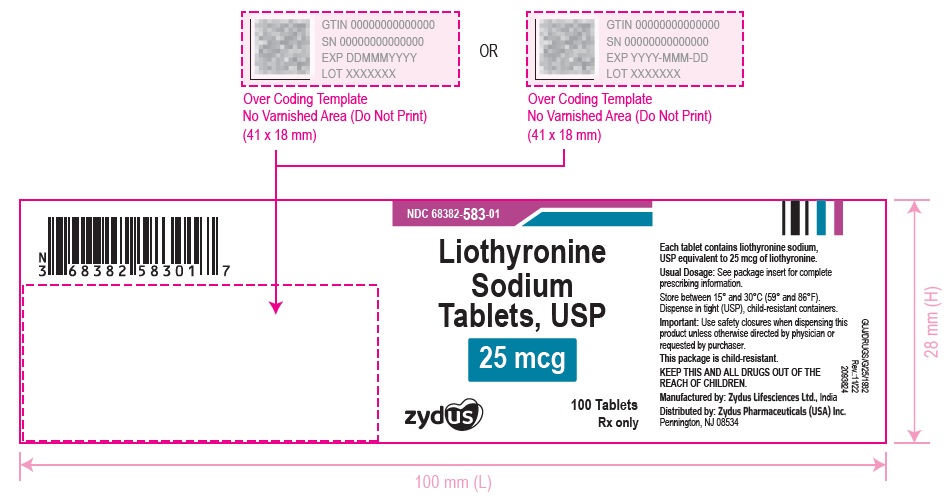
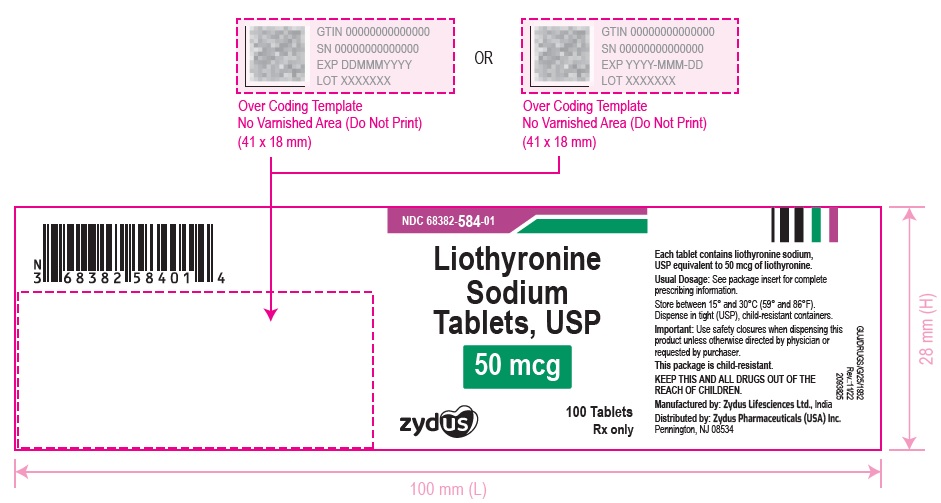
3 DOSAGE FORMS AND STRENGTHSTablets available as follows: 5 mcg: white to off white, round, flat faced beveled edge, uncoated tablets, debossed with '582' on one side and plain on the other side. 25 mcg ...
-
4 CONTRAINDICATIONSLiothyronine sodium is contraindicated in patients with uncorrected adrenal insufficiency [see Warnings and Precautions (5.3)].
-
5 WARNINGS AND PRECAUTIONS5.1 Cardiac Adverse Reactions in the Elderly and in Patients with Underlying Cardiovascular Disease - Overtreatment with thyroid hormone may cause an increase in heart rate, cardiac wall ...
-
6 ADVERSE REACTIONSAdverse reactions associated with liothyronine sodium therapy are primarily those of hyperthyroidism due to therapeutic overdosage [see Warnings and Precautions (5.4) and Overdosage (10)]. They ...
-
7 DRUG INTERACTIONS7.1 Drugs Known to Affect Thyroid Hormone Pharmacokinetics - Many drugs can exert effects on thyroid hormone pharmacokinetics (e.g. absorption, synthesis, secretion, catabolism, protein binding ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Experience with liothyronine use in pregnant women, including data from post-marketing studies, have not reported increased rates of major birth defects or ...
-
10 OVERDOSAGEThe signs and symptoms of overdosage are those of hyperthyroidism [see Warnings and Precautions (5.4) and Adverse Reactions (6)]. In addition, confusion and disorientation may occur. Cerebral ...
-
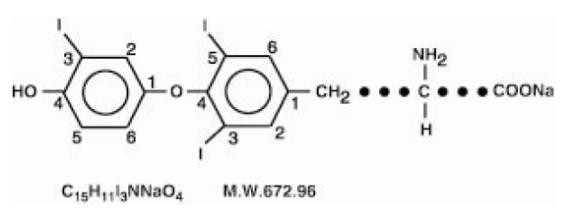
11 DESCRIPTIONLiothyronine sodium tablets, USP contain the active ingredient, liothyronine (L-triiodothyronine or LT3), a synthetic form of a thyroid hormone liothyronine in sodium salt form. It is chemically ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Thyroid hormones exert their physiologic actions through control of DNA transcription and protein synthesis. Triiodothyronine (T3) and L-thyroxine (T4) diffuse into ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Animal studies have not been performed to evaluate the carcinogenic potential, mutagenic potential or effects on fertility of ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGLiothyronine sodium tablets, USP are supplied as follows: 5 mcg tablets are white to off white, round, flat faced beveled edge, uncoated tablets, debossed with '582' on one side and plain on the ...
-
17 PATIENT COUNSELING INFORMATIONDosing and Administration - Instruct patients that liothyronine sodium tablets should only be taken as directed by their healthcare provider. Instruct patients to notify their ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 68382-582-01 - Liothyronine Sodium Tablets, USP - 5 mcg - 100 Tablets - Rx only - Zydus Pharma - NDC 68382-583-01 - Liothyronine Sodium Tablets, USP - 25 mcg - 100 Tablets - Rx only - Zydus ...
-
INGREDIENTS AND APPEARANCEProduct Information