Label: NITROFURANTOIN MACROCRYSTALS capsule
- NDC Code(s): 68382-559-01, 68382-559-10, 68382-559-30, 68382-560-01, view more
- Packager: Zydus Pharmaceuticals (USA) Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 10, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONTo reduce the development of drug-resistant bacteria and maintain the effectiveness of nitrofurantoin macrocrystals capsules and other antibacterial drugs, nitrofurantoin macrocrystals capsules ...
-
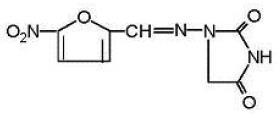
DESCRIPTION:Nitrofurantoin capsules, USP (macrocrystals) is a synthetic chemical of controlled crystal size. It is a stable, lemon-yellow, crystalline compound. Nitrofurantoin capsules, USP (macrocrystals ...
-
CLINICAL PHARMACOLOGY:Nitrofurantoin macrocrystals is a larger crystal form of nitrofurantoin. The absorption of nitrofurantoin macrocrystals is slower and its excretion somewhat less when compared to nitrofurantoin ...
-
MICROBIOLOGYNitrofurantoin is a nitrofuran antimicrobial agent with activity against certain Gram-positive and Gram-negative bacteria. Mechanism of Action - The mechanism of the antimicrobial action of ...
-
INDICATIONS AND USAGE:Nitrofurantoin macrocrystals capsules are specifically indicated for the treatment of urinary tract infections when due to susceptible strains of Escherichia coli, enterococci, Staphylococcus ...
-
CONTRAINDICATIONS:Anuria, oliguria, or significant impairment of renal function (creatinine clearance under 60 mL per minute or clinically significant elevated serum creatinine) are contraindications. Treatment of ...
-
WARNINGS:Pulmonary reactions: ACUTE, SUBACUTE, OR CHRONIC PULMONARY REACTIONS HAVE BEEN OBSERVED IN PATIENTS TREATED WITH NITROFURANTOIN. IF THESE REACTIONS OCCUR, NITROFURANTOIN MACROCRYSTALS SHOULD ...
-
PRECAUTIONS:Information for Patients: Patients should be advised to take nitrofurantoin macrocrystals capsules with food to further enhance tolerance and improve drug absorption. Patients should be ...
-
SPL UNCLASSIFIED SECTIONPregnancy: Teratogenic effects: Several reproduction studies have been performed in rabbits and rats at doses up to 6 times the human dose and have revealed no evidence of impaired fertility or ...
-
ADVERSE REACTIONS:Respiratory: CHRONIC, SUBACUTE, OR ACUTE PULMONARY HYPERSENSITIVITY REACTIONS MAY OCCUR. CHRONIC PULMONARY REACTIONS OCCUR GENERALLY IN PATIENTS WHO HAVE RECEIVED CONTINUOUS TREATMENT FOR ...
-
OVERDOSAGE:Occasional incidents of acute overdosage of nitrofurantoin macrocrystals have not resulted in any specific symptoms other than vomiting. Induction of emesis is recommended. There is no specific ...
-
DOSAGE AND ADMINISTRATION:Nitrofurantoin macrocrystals capsules should be given with food to improve drug absorption and, in some patients, tolerance. Adults: 50 to 100 mg four times a day -- the lower dosage level is ...
-
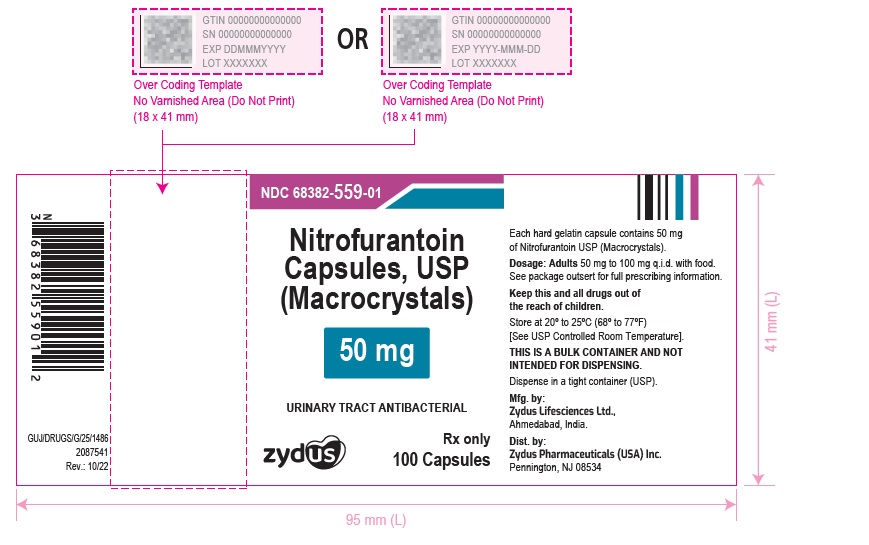
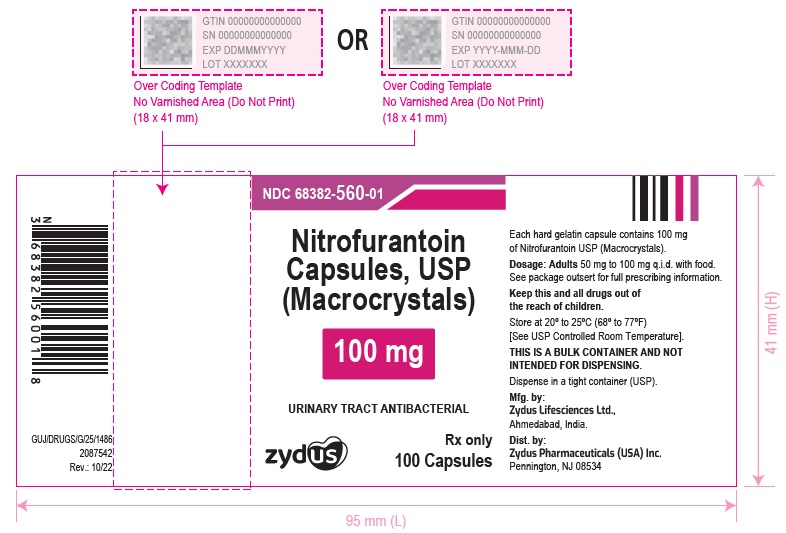
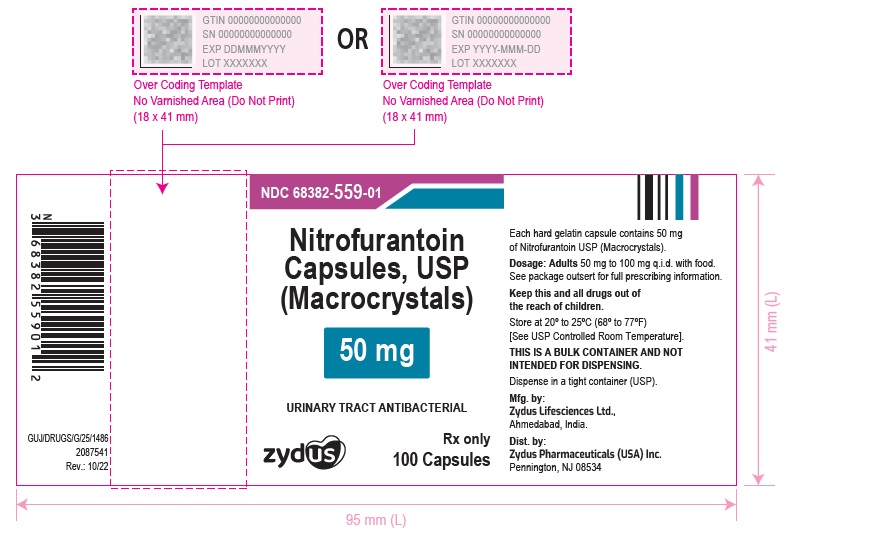
HOW SUPPLIED:Nitrofurantoin Capsules, USP (Macrocrystals) 50 mg are light yellow to yellow powder filled in size "3" empty hard gelatin capsules with blue opaque colored cap and white opaque colored body ...
-
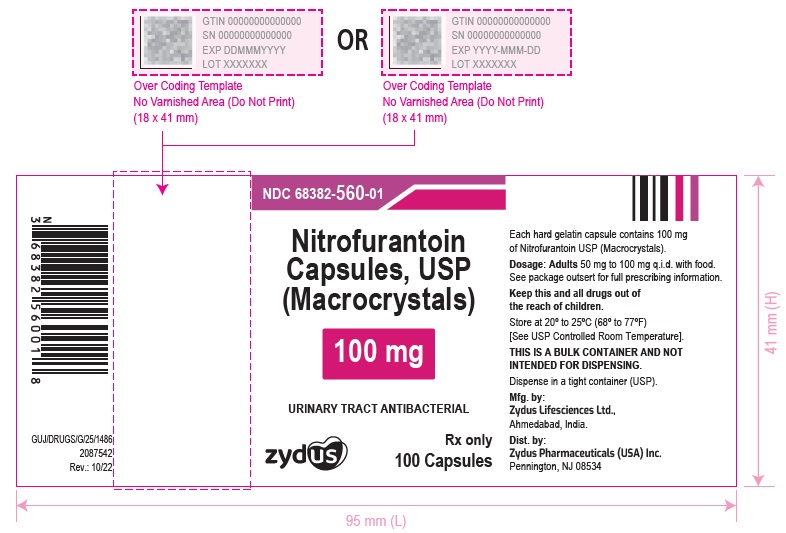
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 68382-559-01 in bottle of 100 Capsules - Nitrofurantoin Capsules, USP 50 mg (Macrocrystals) Rx only - 100 Capsules - NDC 68382-560-01 in bottle of 100 Capsules - Nitrofurantoin Capsules, USP 100 ...
-
INGREDIENTS AND APPEARANCEProduct Information