Label: BUMETANIDE tablet
- NDC Code(s): 68382-525-01, 68382-525-05, 68382-525-06, 68382-525-10, view more
- Packager: Zydus Pharmaceuticals (USA) Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 5, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
Bumetanide is a potent diuretic which, if given in excessive amounts, can lead to a profound diuresis with water and electrolyte depletion. Therefore, careful medical supervision is required, and dose and dosage schedule have to be adjusted to the individual patient's needs (see DOSAGE AND ADMINISTRATION).
Close -
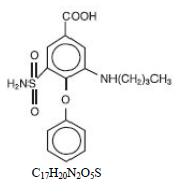
DESCRIPTIONBumetanide is a loop diuretic, available as scored tablets. Chemically, bumetanide is 3-(butylamino)-4-phenoxy-5-sulfamoylbenzoic acid. It is a practically white powder having a calculated ...
-
CLINICAL PHARMACOLOGYBumetanide is a loop diuretic with a rapid onset and short duration of action. Pharmacological and clinical studies have shown that 1 mg bumetanide has a diuretic potency equivalent to ...
-
INDICATIONS AND USAGEBumetanide tablets USP are indicated for the treatment of edema associated with congestive heart failure, hepatic and renal disease, including the nephrotic syndrome. Almost equal diuretic ...
-
WARNINGSVolume and Electrolyte Depletion - The dose of bumetanide should be adjusted to the patient’s need. Excessive doses or too frequent administration can lead to profound water loss, electrolyte ...
-
PRECAUTIONSGeneral - Serum potassium should be measured periodically and potassium supplements or potassium sparing diuretics added if necessary. Periodic determinations of other electrolytes are advised ...
-
ADVERSE REACTIONSThe most frequent clinical adverse reactions considered probably or possibly related to bumetanide are muscle cramps (seen in 1.1% of treated patients), dizziness (1.1%), hypotension (0.8%) ...
-
OVERDOSAGEOverdosage can lead to acute profound water loss, volume and electrolyte depletion, dehydration, reduction of blood volume and circulatory collapse with a possibility of vascular thrombosis and ...
-
DOSAGE AND ADMINISTRATIONIndividualize dosage with careful monitoring of patient response. Oral Administration - The usual total daily dosage of bumetanide tablet is 0.5 mg to 2 mg and in most patients is given as ...
-
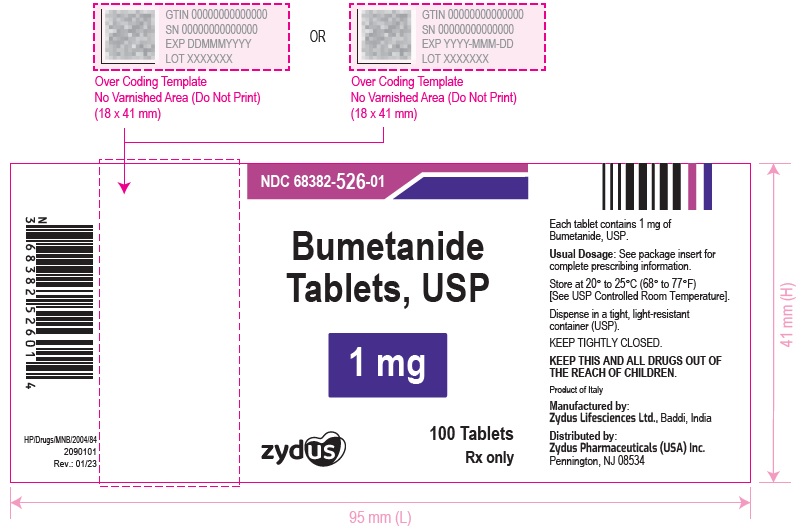
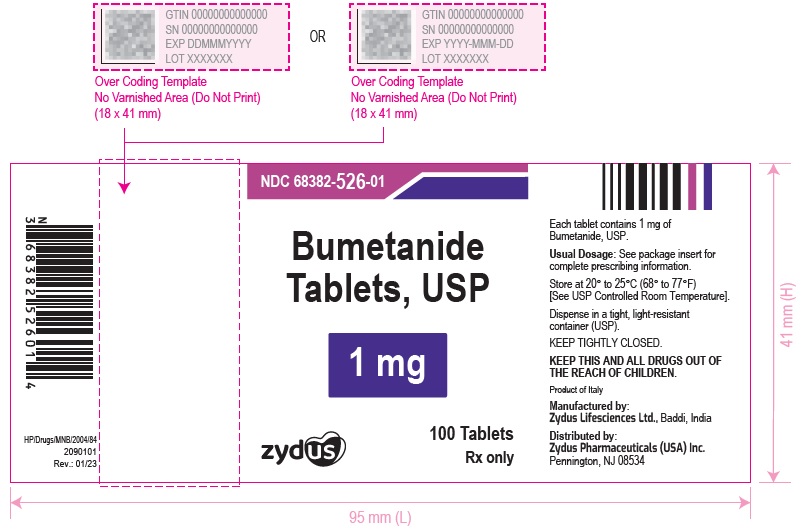
HOW SUPPLIEDBumetanide Tablets USP, 0.5 mg are light green, round, biconvex, uncoated tablet debossed with '525' on one side separating '5' & '25' with breakline and plain on the other side and are supplied ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 68382-525-01 in bottles of 100 tablets - Bumetanide Tablets USP, 0.5 mg - Rx only - 100 Tablets - NDC 68382-526-01 in bottles of 100 tablets - Bumetanide Tablets USP, 1 mg - Rx only - 100 ...
-
INGREDIENTS AND APPEARANCEProduct Information