Label: FAMOTIDINE powder, for suspension
- NDC Code(s): 68382-444-05
- Packager: Zydus Pharmaceuticals USA Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 2, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use FAMOTIDINE FOR ORAL SUSPENSION safely and effectively. See full prescribing information for FAMOTIDINE FOR ORAL ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEFamotidine for oral suspension is indicated in adults for the treatment of: active duodenal ulcer (DU). active gastric ulcer (GU). symptomatic nonerosive gastroesophageal reflux disease ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage in Adults - The recommended dosage and duration of famotidine for oral suspension in adults with normal renal function is shown in Table 1. Table 1: Recommended ...
-
3 DOSAGE FORMS AND STRENGTHSFor Oral Suspension: 400 mg as a white to off-white powder. When constituted as directed, famotidine suspension is a smooth, mobile, off-white, homogeneous suspension with a cherry-mint flavor ...
-
4 CONTRAINDICATIONSFamotidine for oral suspension is contraindicated in patients with a history of serious hypersensitivity reactions (e.g., anaphylaxis) to famotidine or other histamine-2 (H2) receptor ...
-
5 WARNINGS AND PRECAUTIONS5.1 Central Nervous System Adverse Reactions - Central nervous system (CNS) adverse reactions, including confusion, delirium, hallucinations, disorientation, agitation, seizures, and lethargy ...
-
6 ADVERSE REACTIONS6.1 Clinical Trial Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONS7.1 Drugs Dependent on Gastric pH for Absorption - Famotidine can reduce the absorption of other drugs, due to its effect on reducing intragastric acidity, leading to loss of efficacy of the ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available data with H2-receptor antagonists, including famotidine, in pregnant women are insufficient to establish a drug-associated risk of major birth defects ...
-
10 OVERDOSAGEThe types of adverse reactions in overdosage of famotidine are similar to the adverse reactions encountered with use of recommended dosages [see Adverse Reactions (6.1)]. In the event of ...
-
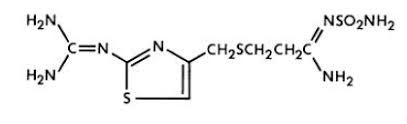
11 DESCRIPTIONThe active ingredient in famotidine for oral suspension is a histamine-2 (H2) receptor antagonist. Famotidine is N'-(aminosulfonyl)-3-[[[2-[(diaminomethylene)amino]-4-thiazolyl]methyl]thio ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Famotidine is a competitive inhibitor of histamine-2 (H2) receptors. The primary clinically important pharmacologic activity of famotidine is inhibition of gastric ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenic potential of famotidine was assessed in a 106-week oral carcinogenicity study in rats and a 92-week oral carcinogenicity ...
-
14 CLINICAL STUDIESThe safety and effectiveness of famotidine for oral suspension have been established based on adequate and well-controlled studies of another oral famotidine product. The following is a summary ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGFamotidine for oral suspension is supplied as follows: NDC - Strength - Quantity - Description - 68382- 444 -05 - 40 mg - Bottle - White to off-white powder. When constituted as ...
-
17 PATIENT COUNSELING INFORMATIONCentral Nervous System (CNS) Adverse Reactions - Advise elderly patients and those with moderate and severe renal impairment of the risk of CNS adverse reactions, including confusion, delirium ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 683825-444-05 - FAMOTIDINE FOR - ORAL SUSPENSION USP - 40 mg/5 ml - 400 mg of famotidine - Constituted to 50 mL, each 5 mL - contains 40 mg famotidine - SHAKE WELL BEFORE USING - NOT FOR ...
-
INGREDIENTS AND APPEARANCEProduct Information