Label: NYSTATIN powder
- NDC Code(s): 68382-370-01, 68382-370-02, 68382-370-03
- Packager: Zydus Pharmaceuticals USA Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 4, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx Only - FOR TOPICAL USE ONLY. NOT FOR OPHTHALMIC USE.
-
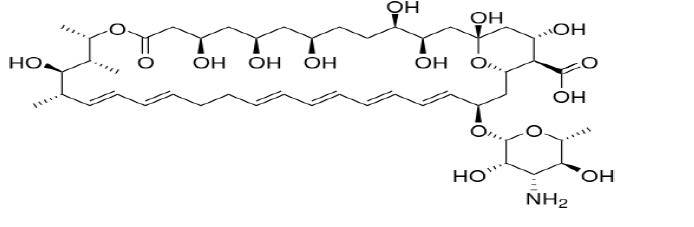
DESCRIPTIONNystatin is a polyene antifungal antibiotic obtained from Streptomyces noursei. Structural formula: Nystatin topical powder is for dermatologic use. Nystatin topical powder contains 100,000 ...
-
CLINICAL PHARMACOLOGYPharmacokinetics - Nystatin is not absorbed from intact skin or mucous membrane. Microbiology - Nystatin is an antibiotic which is both fungistatic and fungicidal in vitro against a wide ...
-
INDICATIONS AND USAGENystatin topical powder is indicated in the treatment of cutaneous or mucocutaneous mycotic infections caused by Candida albicans and other susceptible Candida species. Nystatin topical powder ...
-
CONTRAINDICATIONSNystatin topical powder is contraindicated in patients with a history of hypersensitivity to any of its components.
-
PRECAUTIONSGeneral - Nystatin topical powder should not be used for the treatment of systemic, oral, intravaginal or ophthalmic infections. If irritation or sensitization develops, treatment should be ...
-
ADVERSE REACTIONSThe frequency of adverse events reported in patients using nystatin topical powder is less than 0.1%. The more common events that were reported include allergic reactions, burning, itching ...
-
DOSAGE AND ADMINISTRATIONVery moist lesions are best treated with the topical dusting powder. Adults and Pediatric Patients (Neonates and Older) Apply to candidal lesions two or three times daily until healing is ...
-
HOW SUPPLIEDNystatin topical powder, USP is supplied as 100,000 units nystatin per gram in plastic squeeze bottles: 15 g (NDC 68382-370-01) 30 g (NDC 68382-370-02) 60 g (NDC 68382-370-03) STORAGE - Store ...
-
SPL UNCLASSIFIED SECTIONManufactured By: Zydus Lifesciences Ltd. Baddi, India. Distributed by: Zydus Pharmaceuticals USA Inc. Pennington, NJ 08534 - Rev.: 09/22
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 68382-370-01 - Nystatin Topical Powder, USP - FOR TOPICAL USE ONLY - 100,000 units per gram - Rx Only - 15 GRAMS - Zydus - 15 g Bottle Label
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 68382-370-02 - Nystatin Topical Powder, USP - FOR TOPICAL USE ONLY - 100,000 units per gram - Rx Only - 30 GRAMS - Zydus - 30 g Bottle Label
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 68382-370-03 - Nystatin Topical Powder, USP - FOR TOPICAL USE ONLY - 100,000 units per gram - Rx Only - 60 GRAMS - Zydus - 60 g Bottle Label
-
INGREDIENTS AND APPEARANCEProduct Information