Label: DIPYRIDAMOLE tablet, film coated
- NDC Code(s): 68382-187-01, 68382-187-05, 68382-187-10, 68382-187-77, view more
- Packager: Zydus Pharmaceuticals USA Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 16, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
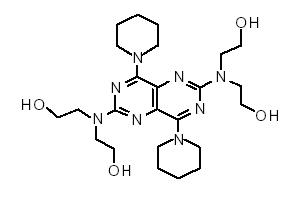
DESCRIPTIONDipyridamole is a platelet inhibitor chemically described as 2,2',2'',2'''-[(4,8-Dipiperidinopyrimido[5,4-d]pyrimidine-2,6-diyl)dinitrilo]-tetraethanol. It has the following structural formula ...
-
CLINICAL PHARMACOLOGYIt is believed that platelet reactivity and interaction with prosthetic cardiac valve surfaces, resulting in abnormally shortened platelet survival time, is a significant factor in thromboembolic ...
-
INDICATIONS AND USAGEDipyridamole tablets are indicated as an adjunct to coumarin anticoagulants in the prevention of postoperative thromboembolic complications of cardiac valve replacement.
-
CONTRAINDICATIONSHypersensitivity to dipyridamole and any of the other components.
-
PRECAUTIONSGeneral - Coronary Artery Disease - Dipyridamole has a vasodilatory effect and should be used with caution in patients with severe coronary artery disease (e.g., unstable angina or recently ...
-
ADVERSE REACTIONSAdverse reactions at therapeutic doses are usually minimal and transient. On long-term use of dipyridamole tablets initial side effects usually disappear. The following reactions in Table 1 were ...
-
OVERDOSAGEIn case of real or suspected overdose, seek medical attention or contact a Poison Control Center immediately. Careful medical management is essential. Based upon the known hemodynamic effects of ...
-
DOSAGE AND ADMINISTRATIONAdjunctive Use in Prophylaxis of Thromboembolism after Cardiac Valve Replacement - The recommended dose is 75 to 100 mg four times daily as an adjunct to the usual warfarin therapy. Please note ...
-
HOW SUPPLIEDDipyridamole Tablets USP, 25 mg are light yellow, round, biconvex, film-coated tablets debossed with 'ZE 43' on one side and plain on the other side are supplied as follows: NDC 68382-187-01 in ...
-
STORAGEStore at 20°C to 25°C (68°F to 77°F) [See USP Controlled Room Temperature]. Keep out of reach of children. Dispense in a tight, light-resistant container. Package insert available at ...
-
SPL UNCLASSIFIED SECTIONManufactured by: Zydus Lifesciences Ltd. Ahmedabad, India - Distributed by: Zydus Pharmaceuticals (USA) Inc. Pennington, NJ 08534 - Rev.: 12/22
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 68382-187-05 in bottle of 500 tablets - Dipyridamole Tablets USP, 25 mg - Rx only - 500 tablets - NDC 68382-188-05 in bottle of 500 tablets - Dipyridamole Tablets USP, 50 mg - Rx only - 500 ...
-
INGREDIENTS AND APPEARANCEProduct Information