Label: PROMETHAZINE HYDROCHLORIDE tablet
- NDC Code(s): 68382-040-01, 68382-040-05, 68382-040-10, 68382-041-01, view more
- Packager: Zydus Pharmaceuticals USA Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 7, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
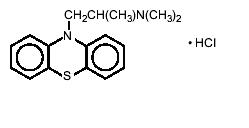
DESCRIPTIONEach tablet of promethazine hydrochloride contains 12.5 mg, 25 mg, or 50 mg promethazine hydrochloride. The inactive ingredients present are hydroxypropyl cellulose, lactose monohydrate ...
-
CLINICAL PHARMACOLOGYPromethazine is a phenothiazine derivative which differs structurally from the antipsychotic phenothiazines by the presence of a branched side chain and no ring substitution. It is thought that ...
-
INDICATIONS AND USAGEPromethazine Hydrochloride, is useful orally for. Perennial and seasonal allergic rhinitis. Vasomotor rhinitis. Allergic conjunctivitis due to inhalant allergens and foods. Mild, uncomplicated ...
-
CONTRAINDICATIONSPromethazine hydrochloride tablets, USP are contraindicated for use in pediatric patients less than two years of age. Promethazine hydrochloride tablets, USP are contraindicated in comatose ...
-
WARNINGSPROMETHAZINE HYDROCHLORIDE TABLETS, USP SHOULD NOT BE USED IN PEDIATRIC PATIENTS LESS THAN 2 YEARS OF AGE BECAUSE OF THE POTENTIAL FOR FATAL RESPIRATORY DEPRESSION. POSTMARKETING CASES OF ...
-
PRECAUTIONSGeneral - Drugs having anticholinergic properties should be used with caution in patients with narrow-angle glaucoma, prostatic hypertrophy, stenosing peptic ulcer, pyloroduodenal obstruction ...
-
ADVERSE REACTIONSCentral Nervous System - Drowsiness is the most prominent CNS effect of this drug. Sedation, somnolence, blurred vision, dizziness; confusion, disorientation, and extrapyramidal symptoms such as ...
-
OVERDOSAGESigns and symptoms of overdosage with promethazine hydrochloride range from mild depression of the central nervous system and cardiovascular system to profound hypotension, respiratory ...
-
DOSAGE AND ADMINISTRATIONPromethazine hydrochloride tablets, USP are contraindicated for children under 2 years of age (see WARNINGS-Black Box Warning and Use in Pediatric Patients). Allergy - The average oral dose is ...
-
HOW SUPPLIEDPromethazine hydrochloride tablets USP, 12.5 mg are white to off-white, round shape, biconvex, uncoated tablets debossed with the logo of "ZC", "01" and bisect on one side and plain on the other ...
-
MEDICATION GUIDEPatient Information Leaflet - Promethazine Hydrochloride Tablets, USP - (proe meth′ a zeen hye″ droe klor′ ide) This is a summary of the most important information about promethazine. For details ...
-
SPL UNCLASSIFIED SECTIONManufactured by: Zydus Lifesciences Ltd. India - Distributed by: Zydus Pharmaceuticals USA Inc. Pennington, NJ 08534 - Rev.: 09/22
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 68382-040-01 in bottle of 100 tablets - Promethazine Hydrochloride Tablets USP, 12.5 mg - Rx only - 100 tablets - ZYDUS - NDC 68382-041-01 in bottle of 100 tablets - Promethazine Hydrochloride Tablets ...
-
INGREDIENTS AND APPEARANCEProduct Information