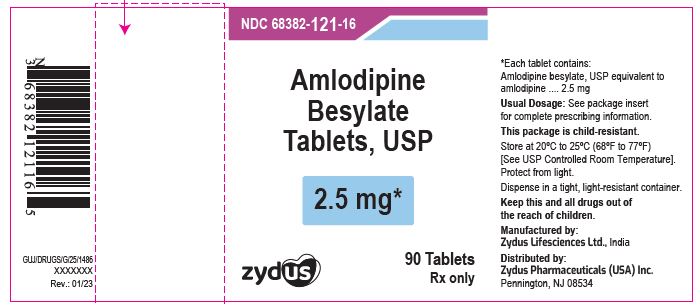
Label: AMLODIPINE BESYLATE tablet
- NDC Code(s): 68382-121-01, 68382-121-05, 68382-121-16, 68382-121-77, view more
- Packager: Zydus Pharmaceuticals USA Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use AMLODIPINE BESYLATE TABLETSsafely and effectively. See full prescribing information for AMLODIPINE BESYLATE TABLETS. AMLODIPINE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Hypertension - Amlodipine besylate tablets, USP are indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal ...
-
2 DOSAGE AND ADMINISTRATION2.1 Adults - The usual initial antihypertensive oral dose of amlodipine besylate tablets is 5 mg once daily, and the maximum dose is 10 mg once daily. Small, fragile, or elderly patients, or ...
-
3 DOSAGE FORMS AND STRENGTHSAmlodipine Besylate Tablets, USP equivalent to 2.5 mg of amlodipine are supplied as white to off-white, round, flat, radial-edged tablets debossed with 'Z' on one side and '7' on the other ...
-
4 CONTRAINDICATIONSAmlodipine besylate tabets, USP are contraindicated in patients with known sensitivity to amlodipine.
-
5 WARNINGS AND PRECAUTIONS5.1 Hypotension - Symptomatic hypotension is possible, particularly in patients with severe aortic stenosis. Because of the gradual onset of action, acute hypotension is unlikely. 5.2 ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONS7.1 Impact of Other Drugs on Amlodipine - CYP3A Inhibitors - Coadministration with CYP3A inhibitors (moderate and strong) results in increased systemic exposure to amlodipine and may require ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - The limited available data based on post-marketing reports with amlodipine besylate use in pregnant women are not sufficient to inform a drug-associated risk for ...
-
10 OVERDOSAGEOverdosage might be expected to cause excessive peripheral vasodilation with marked hypotension and possibly a reflex tachycardia. In humans, experience with intentional overdosage of amlodipine ...
-
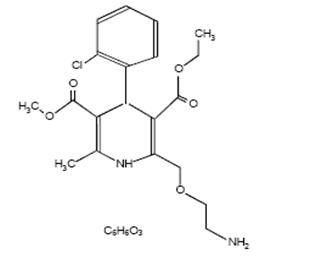
11 DESCRIPTIONAmlodipine besylate is the besylate salt of amlodipine, a long-acting calcium channel blocker. Amlodipine besylate is chemically described as 3-Ethyl-5-methyl ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Amlodipine is a dihydropyridine calcium antagonist (calcium ion antagonist or slow-channel blocker) that inhibits the transmembrane influx of calcium ions into ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Rats and mice treated with amlodipine maleate in the diet for up to two years, at concentrations calculated to provide daily dosage ...
-
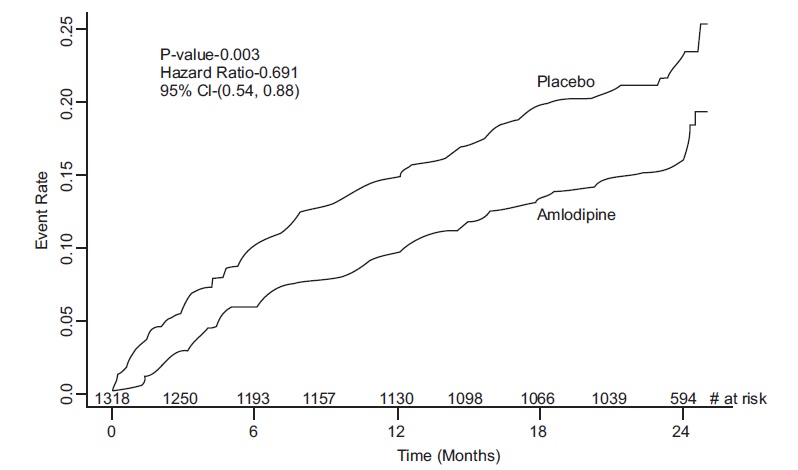
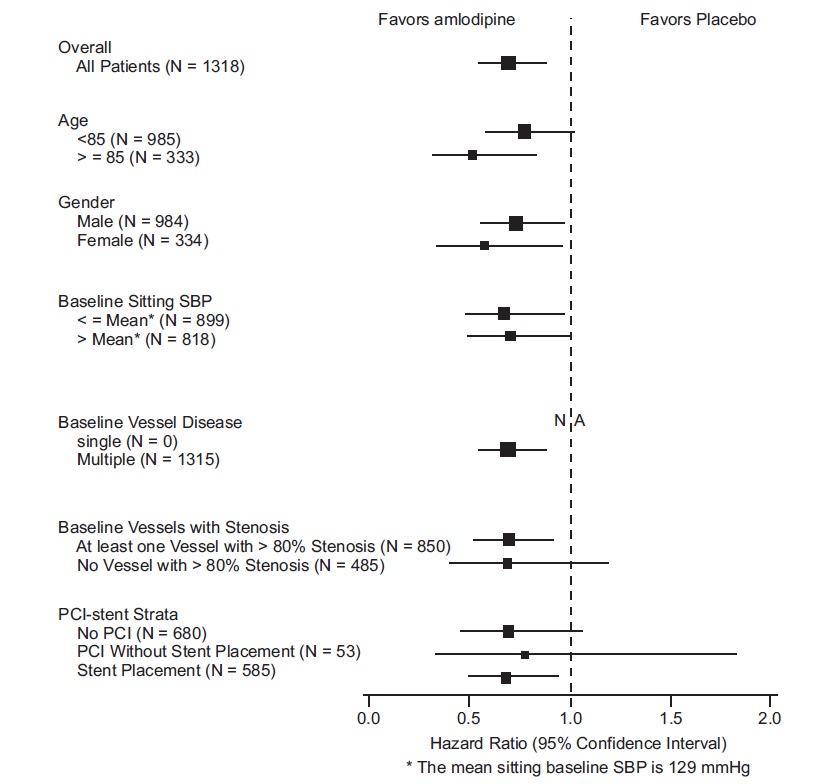
14 CLINICAL STUDIES14.1 Effects in Hypertension - Adult Patients - The antihypertensive efficacy of amlodipine besylate has been demonstrated in a total of 15 double-blind, placebo-controlled, randomized studies ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGAmlodipine Besylate Tablets, USP equivalent to 2.5 mg of amlodipine are supplied as white to off-white, round, flat, radial-edged tablets debossed with 'Z' on one side and '7' on the other side ...
-
SPL UNCLASSIFIED SECTIONManufactured by: Zydus Lifesciences Ltd. India - Distributed by: Zydus Pharmaceuticals (USA) Inc. Pennington, NJ 08534 - Rev.: 01/23
-
PATIENT PACKAGE INSERTPatient Information Leaflet - Amlodipine Besylate - (am loe′ di peen bes′ i late) Tablets, USP - Read this information carefully before you start taking amlodipine besylate tablets and each time ...
-
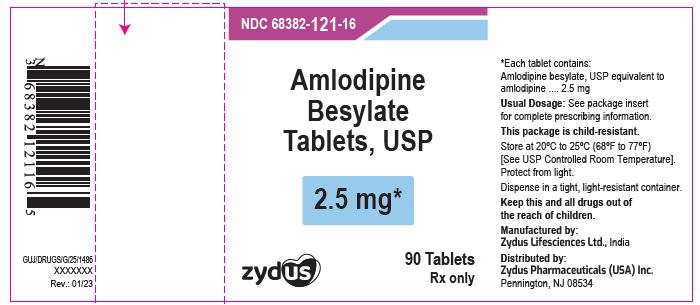
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 68382-121-16 in bottle of 90 tablets - Amlodipine Besylate Tablets USP, 2.5 mg - Rx only - 90 tablets - ZYDUS - NDC 68382-122-16 in bottle of 90 tablets - Amlodipine Besylate Tablets USP, 5 mg - Rx ...
-
INGREDIENTS AND APPEARANCEProduct Information