Label: DROXIDOPA capsule
- NDC Code(s): 68180-987-02, 68180-987-09, 68180-988-02, 68180-988-09, view more
- Packager: Lupin Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 18, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use DROXIDOPA CAPSULES safely and effectively. See full prescribing information for DROXIDOPA CAPSULES. DROXIDOPA capsules, for oral ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: SUPINE HYPERTENSION
Monitor supine blood pressure prior to and during treatment and more frequently when increasing doses. Elevating the head of the bed lessens the risk of supine hypertension, and blood pressure should be measured in this position. If supine hypertension cannot be managed by elevation of the head of the bed, reduce or discontinue droxidopa [see Warnings and Precautions (5.1)].
Close -
1 INDICATIONS AND USAGEDroxidopa capsules are indicated for the treatment of orthostatic dizziness, lightheadedness, or the "feeling that you are about to black out" in adult patients with symptomatic neurogenic ...
-
2 DOSAGE AND ADMINISTRATION2.1 Dosing Information - The recommended starting dose of droxidopa capsules are 100 mg, taken orally three times daily: upon arising in the morning, at midday, and in the late afternoon at ...
-
3 DOSAGE FORMS AND STRENGTHSDroxidopa capsules are available in 100 mg, 200 mg, and 300 mg strengths as specified below. 100 mg: Hard gelatin, size 3 capsule, with an opaque light blue cap and an opaque white ...
-
4 CONTRAINDICATIONSDroxidopa capsules are contraindicated in patients who have a history of hypersensitivity to the drug or its ingredients [see Warnings and Precautions (5.4)].
-
5 WARNINGS AND PRECAUTIONS5.1 Supine Hypertension - Droxidopa therapy may cause or exacerbate supine hypertension in patients with nOH. Patients should be advised to elevate the head of the bed when resting or sleeping ...
-
6 ADVERSE REACTIONSThe following adverse reactions with droxidopa capsules are included in more detail in the Warnings and Precautions section of the label: Supine Hypertension [see Warnings and ...
-
7 DRUG INTERACTIONS7.1 Drugs that Increase Blood Pressure - Administering droxidopa in combination with other agents that increase blood pressure (e.g., norepinephrine, ephedrine, midodrine, and triptans) would be ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no available data on use of droxidopa in pregnant women and risk of major birth defects or miscarriage. Droxidopa did not produce significant ...
-
10 OVERDOSAGE10.1 Symptoms - There have been cases of overdose reported during postmarketing surveillance. A patient ingested 7,700 mg of droxidopa and experienced a hypertensive crisis that resolved ...
-
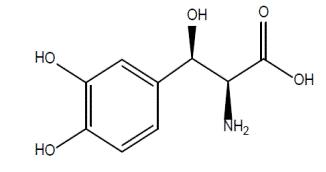
11 DESCRIPTIONDroxidopa capsules contain droxidopa, which is a synthetic amino acid precursor of norepinephrine, for oral administration. Chemically, droxidopa is (–)-threo-3-(3,4 Dihydroxyphenyl)-L-serine. It ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The exact mechanism of action of droxidopa in the treatment of neurogenic orthostatic hypotension is unknown. Droxidopa is a synthetic amino acid analog that is ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term studies have been conducted at dosages up to 1,000 mg/kg/day in mice and up to 100 mg/kg/day in rats with no indication of ...
-
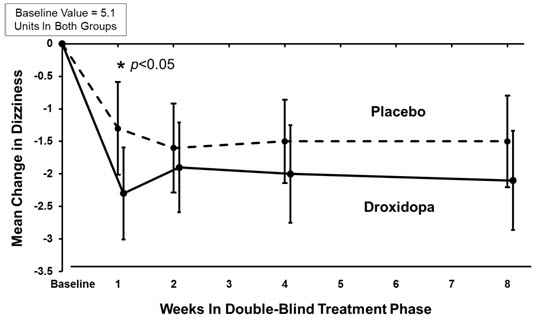
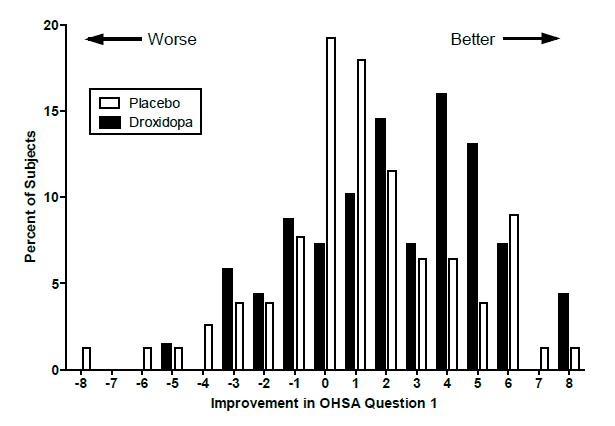
14 CLINICAL STUDIES14.1 Studies in Neurogenic Orthostatic Hypotension - Clinical studies (described below) examined the efficacy of droxidopa in the short-term (1 to 2 weeks) and over longer-term periods (8 weeks ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - Droxidopa capsules are supplied in the following dosage strengths: 100 mg: Hard gelatin, size 3 capsule, with an opaque light blue cap and an opaque white body, printed with ...
-
17 PATIENT COUNSELING INFORMATIONElevations in Blood Pressure - Counsel patients that Droxidopa causes elevations in blood pressure and increases the risk of supine hypertension, which could lead to strokes, heart attacks, and ...
-
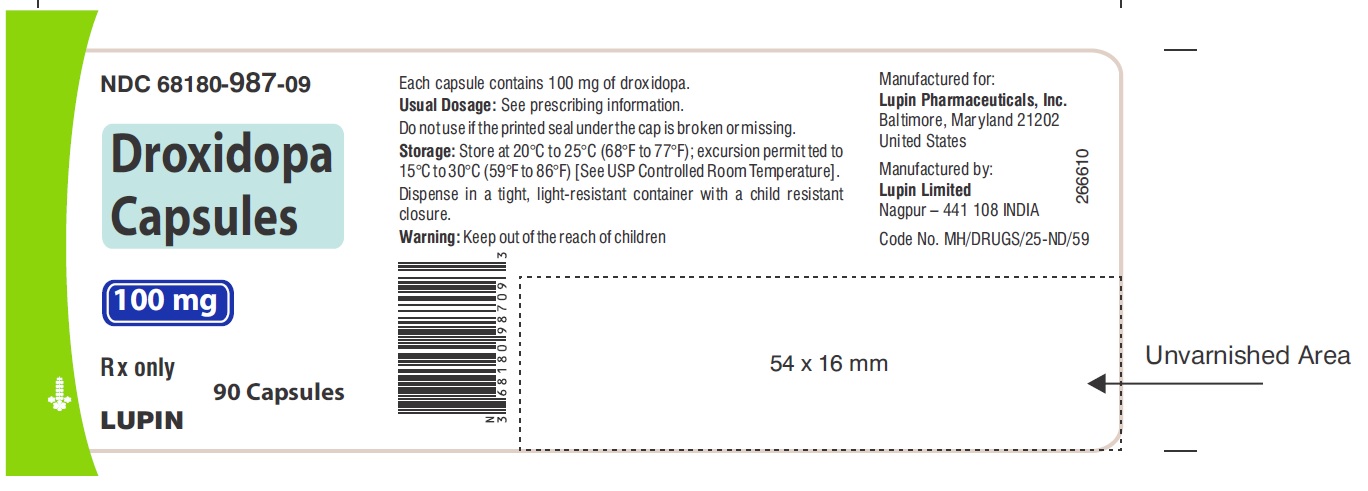
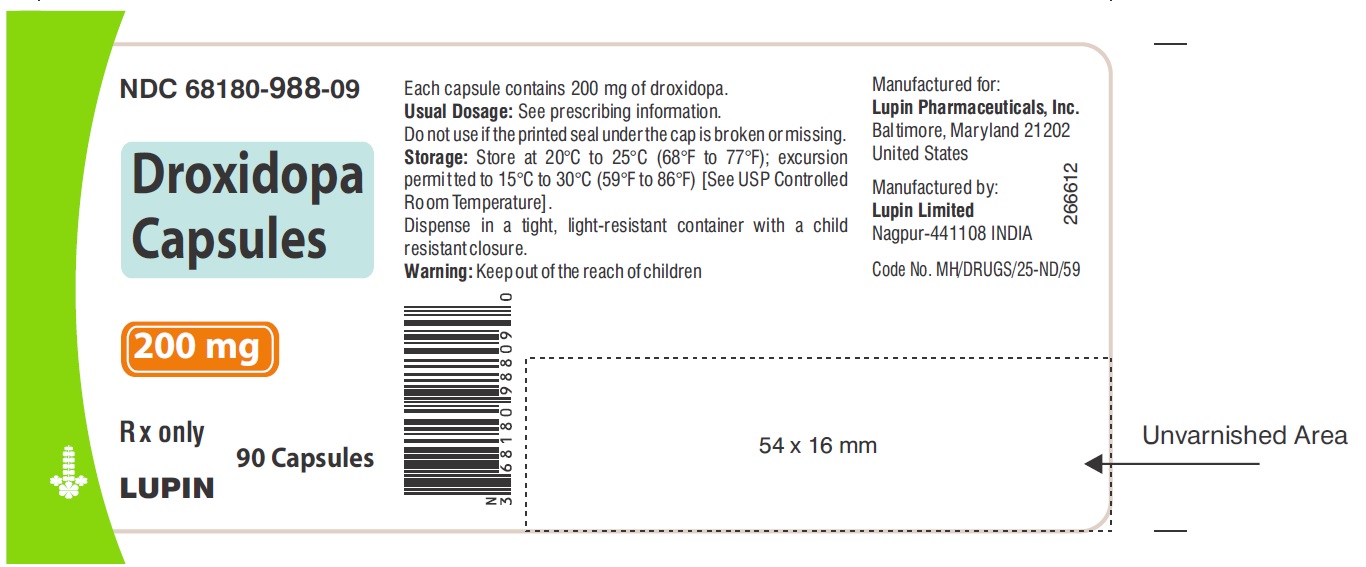
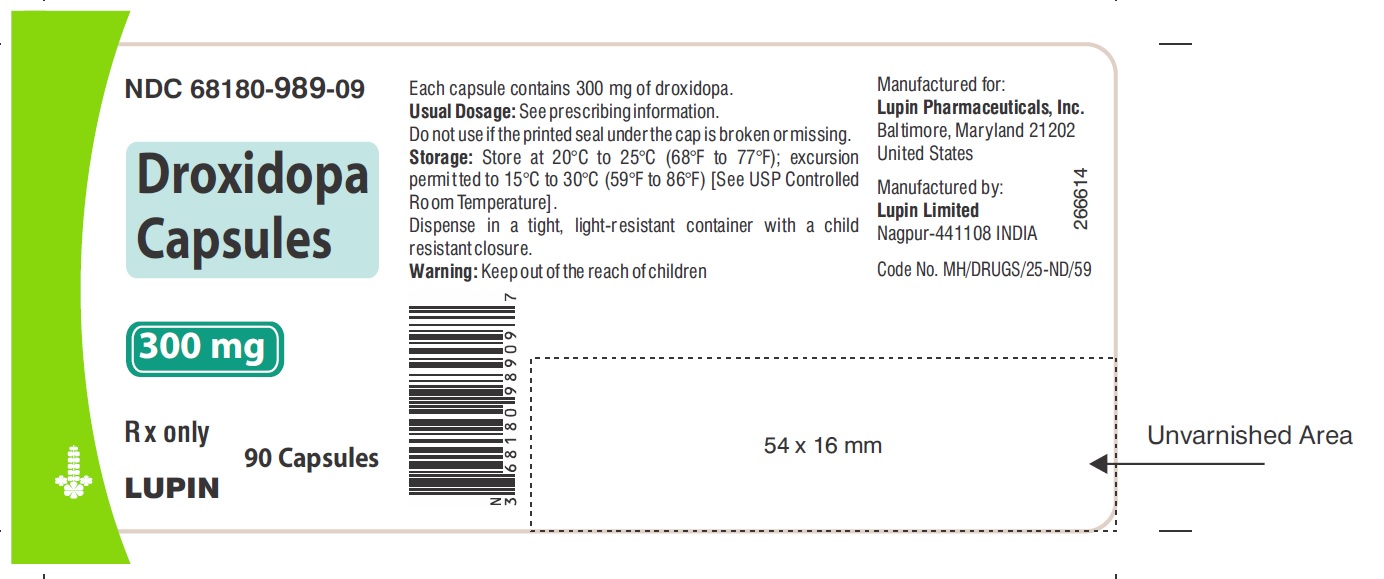
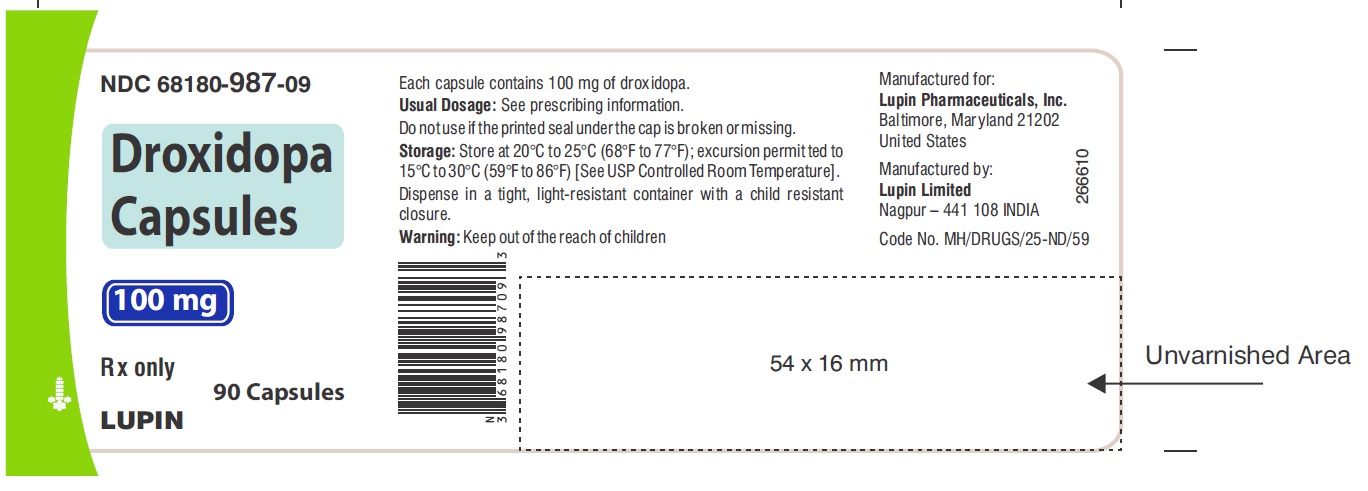
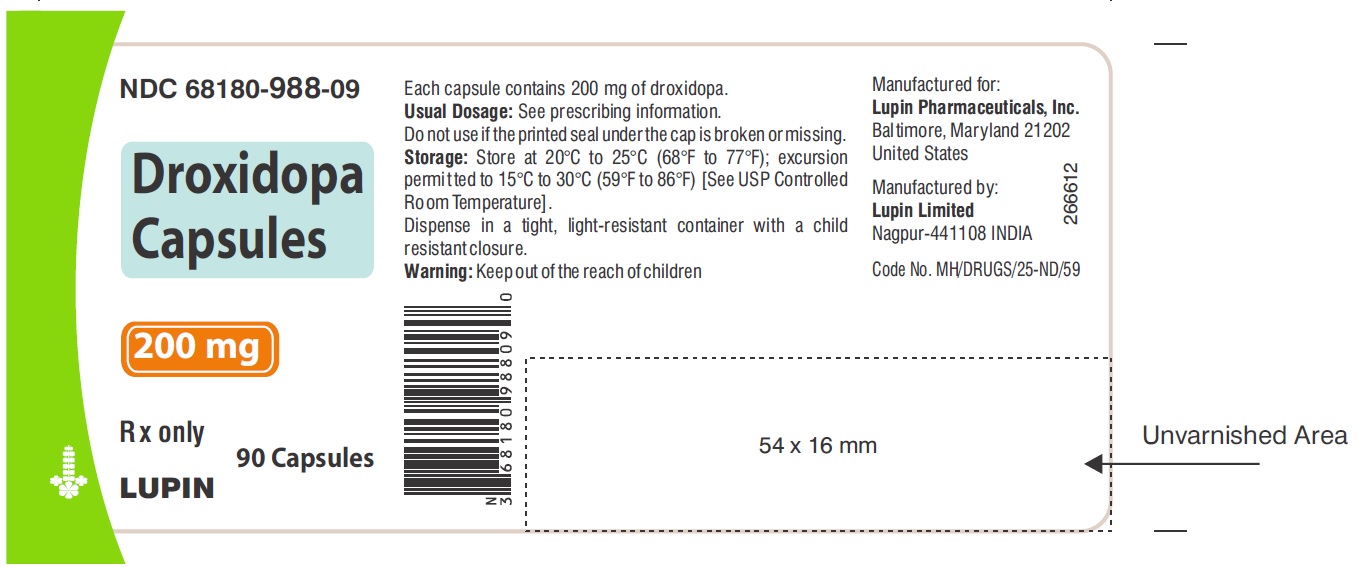
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 68180-987-09 - Droxidopa Capsules - 100 mg - Bottle of 90's Capsules - NDC 68180-988-09 - Droxidopa Capsules - 200 mg - Bottle of 90's Capsules - NDC 68180-989-09 - Droxidopa Capsules - 300 mg - Bottle of ...
-
INGREDIENTS AND APPEARANCEProduct Information