Label: AZITHROMYCIN DIHYDRATE tablet, film coated
-
NDC Code(s):
68180-861-06,
68180-861-11,
68180-861-13,
68180-862-06, view more68180-862-11, 68180-862-13
- Packager: Lupin Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 21, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use AZITHROMYCIN TABLETS safely and effectively. See full prescribing information for AZITHROMYCIN TABLETS. AZITHROMYCIN tablets, 250 mg ...These highlights do not include all the information needed to use AZITHROMYCIN TABLETS safely and effectively. See full prescribing information for AZITHROMYCIN TABLETS.
AZITHROMYCIN tablets, 250 mg and 500 mg, for oral use
Initial U.S. Approval: 1991RECENT MAJOR CHANGES
Warnings and Precautions, Cardiovascular Death (5.5) 11/2021
INDICATIONS AND USAGE
Azithromycin tablets are a macrolide antibacterial drug indicated for mild to moderate infections caused by designated, susceptible bacteria:
- Acute bacterial exacerbations of chronic bronchitis in adults (1.1)
- Acute bacterial sinusitis in adults (1.1)
- Uncomplicated skin and skin structure infections in adults (1.1)
- Urethritis and cervicitis in adults (1.1)
- Genital ulcer disease in men (1.1)
- Acute otitis media in pediatric patients (6 months of age and older) (1.2)
- Community-acquired pneumonia in adults and pediatric patients (6 months of age and older) (1.1, 1.2)
- Pharyngitis/tonsillitis in adults and pediatric patients (2 years of age and older) (1.1, 1.2)
Limitation of Use:
Azithromycin should not be used in patients with pneumonia who are judged to be inappropriate for oral therapy because of moderate to severe illness or risk factors. (1.3)
To reduce the development of drug-resistant bacteria and maintain the effectiveness of azithromycin tablets and other antibacterial drugs, azithromycin tablets should be used only to treat infections that are proven or strongly suspected to be caused by susceptible bacteria. (1.4)
DOSAGE AND ADMINISTRATION
- Adult Patients (2.1)
Infection
Recommended Dose/Duration of Therapy
Community-acquired pneumonia (mild severity)
Pharyngitis/tonsillitis (second-line therapy)
Skin/skin structure (uncomplicated)
500 mg as a single dose on Day 1, followed by 250 mg once daily on Days 2 through 5.
Acute bacterial exacerbations of chronic bronchitis (mild to moderate)
500 mg as a single dose on Day 1, followed by 250 mg once daily on Days 2 through 5 or 500 mg once daily for 3 days.
Acute bacterial sinusitis
500 mg once daily for 3 days.
Genital ulcer disease (chancroid) Non-gonococcal urethritis and cervicitis
One single 1 gram dose.
Gonococcal urethritis and cervicitis
One single 2 gram dose.
- Pediatric Patients (2.2)
Infection
Recommended Dose/Duration of Therapy
Acute otitis media (6 months of age and older)
30 mg/kg as a single dose or 10 mg/kg once daily for 3 days or 10 mg/kg as a single dose on Day 1 followed by 5 mg/kg/day on Days 2 through 5.
Acute bacterial sinusitis (6 months of age and older)
10 mg/kg once daily for 3 days.
Community-acquired pneumonia (6 months of age and older)
10 mg/kg as a single dose on Day 1 followed by 5 mg/kg once daily on Days 2 through 5.
Pharyngitis/tonsillitis (2 years of age and older)
12 mg/kg once daily for 5 days.
DOSAGE FORMS AND STRENGTHS
- Azithromycin tablets, 250 mg and 500 mg (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Serious (including fatal) allergic and skin reactions: Discontinue azithromycin if reaction occurs. (5.1)
- Hepatotoxicity: Severe, and sometimes fatal, hepatotoxicity has been reported. Discontinue azithromycin immediately if signs and symptoms of hepatitis occur. (5.2)
- Infantile Hypertrophic Pyloric Stenosis (IHPS): Following the use of azithromycin in neonates (treatment up to 42 days of life), IHPS has been reported. Direct parents and caregivers to contact their physician if vomiting or irritability with feeding occurs. (5.3)
- Prolongation of QT interval and cases of torsades de pointes have been reported. This risk which can be fatal should be considered in patients with certain cardiovascular disorders including known QT prolongation or history torsades de pointes, those with proarrhythmic conditions, and with other drugs that prolong the QT interval. (5.4)
- Cardiovascular Death: Some observational studies have shown an approximately two-fold increased short-term potential risk of acute cardiovascular death in adults exposed to azithromycin relative to other antibacterial drugs, including amoxicillin. Consider balancing this potential risk with treatment benefits when prescribing Azithromycin tablets. (5.5)
- Clostridioides difficile -Associated Diarrhea: Evaluate patients if diarrhea occurs.(5.6)
- Azithromycin may exacerbate muscle weakness in persons with myasthenia gravis. (5.7)
ADVERSE REACTIONS
Most common adverse reactions are diarrhea (5 to 14%), nausea (3 to 18%), abdominal pain (3 to 7%), or vomiting (2 to 7%). (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Lupin Pharmaceuticals, Inc. at 1-800-399-2561 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 2/2025
Close -
Table of ContentsTable of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Adult Patients
1.2 Pediatric Patients
1.3 Limitations of Use
1.4 Usage
2 DOSAGE AND ADMINISTRATION
2.1 Adult Patients
2.2 Pediatric Patients
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Hypersensitivity
4.2 Hepatic Dysfunction
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity
5.2 Hepatotoxicity
5.3 Infantile Hypertrophic Pyloric Stenosis (IHPS)
5.4 QT Prolongation
5.5 Cardiovascular Death
5.6 Clostridioides difficile-Associated Diarrhea
5.7 Exacerbation of Myasthenia Gravis
5.8 Use in Sexually Transmitted Disease
5.9 Development of Drug-Resistant Bacteria
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
6.3 Laboratory Abnormalities
7 DRUG INTERACTIONS
7.1 Nelfinavir
7.2 Warfarin
7.3 Potential Drug-Drug Interaction with Macrolides
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Adult Patients
14.2 Pediatric Patients
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGEAzithromycin tablets are a macrolide antibacterial drug indicated for the treatment of patients with mild to moderate infections caused by susceptible strains of the designated microorganisms ...
Azithromycin tablets are a macrolide antibacterial drug indicated for the treatment of patients with mild to moderate infections caused by susceptible strains of the designated microorganisms in the specific conditions listed below. Recommended dosages and durations of therapy in adult and pediatric patient populations vary in these indications. [see DOSAGE AND ADMINISTRATION (2)]
1.1 Adult Patients
- Acute bacterial exacerbations of chronic bronchitis due to Haemophilus influenzae , Moraxella catarrhalis, or Streptococcus pneumoniae .
- Acute bacterial sinusitis due to Haemophilus influenzae , Moraxella catarrhalis or Streptococcus pneumoniae .
- Community-acquired pneumonia due to Chlamydophila pneumoniae , Haemophilus influenzae , Mycoplasma pneumoniae, or Streptococcus pneumoniae in patients appropriate for oral therapy.
- Pharyngitis/tonsillitis caused by Streptococcus pyogenes as an alternative to first-line therapy in individuals who cannot use first-line therapy.
- Uncomplicated skin and skin structure infections due to Staphylococcus aureus , Streptococcus pyogenes , or Streptococcus agalactiae .
- Urethritis and cervicitis due to Chlamydia trachomatis or Neisseria gonorrhoeae .
- Genital ulcer disease in men due to Haemophilus ducreyi (chancroid). Due to the small number of women included in clinical trials, the efficacy of azithromycin in the treatment of chancroid in women has not been established.
1.2 Pediatric Patients
[see USE IN SPECIFIC POPULATIONS (8.4) and CLINICAL STUDIES (14.2)]
- Acute otitis media (>6 months of age) caused by Haemophilus influenzae , Moraxella catarrhalis, or Streptococcus pneumoniae.
- Community-acquired pneumonia (>6 months ofage) due to Chlamydophila pneumoniae , Haemophilus influenzae , Mycoplasma pneumoniae , or Streptococcus pneumoniae in patients appropriate for oral therapy.
- Pharyngitis/tonsillitis (>2 years of age) caused by Streptococcus pyogenes as an alternative to first-line therapy in individuals who cannot use first-line therapy.
1.3 Limitations of Use
Azithromycin should not be used in patients with pneumonia who are judged to be inappropriate for oral therapy because of moderate to severe illness or risk factors such as any of the following:
- patients with cystic fibrosis,
- patients with nosocomial infections,
- patients with known or suspected bacteremia,
- patients requiring hospitalization,
- elderly or debilitated patients, or
- patients with significant underlying health problems that may compromise their ability to respond to their illness (including immunodeficiency or functional asplenia).
Close1.4 Usage
To reduce the development of drug-resistant bacteria and maintain the effectiveness of azithromycin and other antibacterial drugs, azithromycin should be used only to treat infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
-
2 DOSAGE AND ADMINISTRATION2.1 Adult Patients - [see INDICATIONS AND USAGE (1.1) and CLINICAL PHARMACOLOGY (12.3)] Infection* Recommended Dose/Duration of Therapy - Community-acquired pneumonia ...
2.1 Adult Patients
[see INDICATIONS AND USAGE (1.1) and CLINICAL PHARMACOLOGY (12.3)]
Infection*
Recommended Dose/Duration of Therapy
Community-acquired pneumonia Pharyngitis/tonsillitis (second-line therapy) Skin/skin structure (uncomplicated)
500 mg as a single dose on Day 1, followed by 250 mg once daily on Days 2 through 5
Acute bacterial exacerbations of chronic obstructive pulmonary disease
500 mg once daily for 3 days
OR
500 mg as a single dose on Day 1, followed by 250 mg once daily on Days 2 through 5
Acute bacterial sinusitis
500 mg once daily for 3 days
Genital ulcer disease (chancroid)
One single 1 gram dose
Non-gonococcal urethritis and cervicitis
One single 1 gram dose
Gonococcal urethritis and cervicitis
One single 2 gram dose
*DUE TO THE INDICATED ORGANISMS [see INDICATIONS AND USAGE (1.1)]
Close2.2 Pediatric Patients
Infection*
Recommended Dose/Duration of Therapy
Acute otitis media
30 mg/kg as a single dose or 10 mg/kg once daily for 3 days or 10 mg/kg as a single dose on Day 1 followed by 5 mg/kg/day on Days 2 through 5.
Acute bacterial sinusitis
10 mg/kg once daily for 3 days.
Community-acquired pneumonia
10 mg/kg as a single dose on Day 1 followed by 5 mg/kg once daily on Days 2 through 5.
Pharyngitis/tonsillitis
12 mg/kg once daily for 5 days.
*DUE TO THE INDICATED ORGANISMS [see INDICATIONS AND USAGE (1.2)] 1 see dosing tables below for maximum doses evaluated by indication
Azithromycin for oral suspension can be taken with or without food.
PEDIATRIC DOSAGE GUIDELINES FOR OTITIS MEDIA, ACUTE BACTERIAL SINUSITIS, AND COMMUNITY-ACQUIRED PNEUMONIA (Age 6 months and above, [see USE IN SPECIFIC POPULATIONS (8.4)] ) Based on Body Weight * Effectiveness of the 3-day or 1-day regimen in pediatric patients with community-acquired pneumonia has not been established.
OTITIS MEDIA AND COMMUNITY-ACQUIRED PNEUMONIA: (5-Day Regimen)*
Dosing Calculated on 10 mg/kg/day Day 1 and 5 mg/kg/day Days 2 to 5.
Weight
100 mg/5 mL
200 mg/5 mL
Total mL per Treatment Course
Total mg per Treatment Course
Kg
Day 1
Days 2 to 5
Day 1
Days 2 to 5
5
2.5 mL; (½ tsp)
1.25 mL;(¼ tsp)
7.5 mL
150 mg
10
5 mL; (1tsp)
2.5 mL; (½ tsp)
15 mL
300 mg
20
5 mL; (1 tsp)
2.5 mL; (½ tsp)
15 mL
600 mg
30
7.5 mL; (1½ tsp)
3.75 mL; (¾ tsp)
22.5 mL
900 mg
40
10 mL; (2 tsp)
5 mL;(1 tsp)
30 mL
1200 mg
50 and above
12.5 mL; (2½ tsp)
6.25 mL; (1¼ tsp)
37.5 mL
1500 mg
* Effectiveness of the 5-day or 1-day regimen in pediatric patients with acute bacterial sinusitis has not been established.
OTITIS MEDIA AND ACUTE BACTERIAL SINUSITIS: (3-Day Regimen)*
Dosing Calculated on 10 mg/kg/day.
Weight
100 mg/5 mL
200 mg/5 mL
Kg
Days 1 to 3
Days 1 to 3
Total mL per
Treatment Course
Total mg per Treatment Course
5
2.5 mL; (1/2 tsp)
7.5 mL
150 mg
10
5 mL; (1 tsp)
15 mL
300 mg
20
5 mL (1 tsp)
15 mL
600 mg
30
7.5 mL (1½ tsp)
22.5 mL
900 mg
40
10 mL (2 tsp)
30 mL
1200 mg
50 and above
12.5 mL (2 ½ tsp)
37.5 mL
1500 mg
OTITIS MEDIA: (1-Day Regimen)
Dosing Calculated on 30 mg/kg as a single dose.
Weight
200 mg/5 mL
Total mL per Treatment Course
Total mg per Treatment Course
Kg
1-Day Regimen
5
3.75 mL;(3/4 tsp)
3.75 mL
150 mg
10
7.5 mL;(1½ tsp)
7.5 mL
300 mg
20
15 mL;(3 tsp)
15 mL
600 mg
30
22.5 mL;(4½ tsp)
22.5 mL
900 mg
40
30 mL;(6 tsp)
30 mL
1200 mg
50 and above
37.5 mL;(7½ tsp)
37.5 mL
1500 mg
The safety of re-dosing azithromycin in pediatric patients who vomit after receiving 30 mg/kg as a single dose has not been established. In clinical studies involving 487 patients with acute otitis media given a single 30 mg/kg dose of azithromycin, 8 patients who vomited within 30 minutes of dosing were re-dosed at the same total dose.
Pharyngitis/Tonsillitis
The recommended dose of azithromycin for children with pharyngitis/tonsillitis is 12 mg/kg once daily for 5 days. (See chart below.)
PEDIATRIC DOSAGE GUIDELINES FOR PHARYNGITIS/TONSILLITIS (Age 2 years and above, [see USE IN SPECIFIC POPULATIONS (8.4)] ) Based on Body Weight PHARYNGITIS/TONSILLITIS: (5-Day Regimen)
Dosing Calculated on 12 mg/kg/day for 5 days.
Weight
200 mg/5 mL
Total mL per Treatment Course
Total mg per Treatment Course
Kg
Day 1 to 5
8
2.5 mL; (½ tsp)
12.5 mL
500 mg
17
5 mL; (1 tsp)
25 mL
1000 mg
25
7.5 mL; (1½ tsp)
37.5 mL
1500 mg
33
10 mL; (2 tsp)
50 mL
2000 mg
40
12.5 mL; (2½ tsp)
62.5 mL
2500 mg
Constituting instructions for azithromycin oral suspension 300, 600, 900, 1200 mg bottles. The table below indicates the volume of water to be used for constitution:
Amount of water to be added
Total volume after constitution (azithromycin content)
Azithromycin concentration after constitution
9 mL (300 mg)
15 mL (300 mg)
100 mg/5 mL
9 mL (600 mg)
15 mL (600 mg)
200 mg/5 mL
12 mL (900 mg)
22.5 mL (900 mg)
200 mg/5 mL
15 mL (1200 mg)
30 mL (1200 mg)
200 mg/5 mL
Shake well before each use. Oversized bottle provides shake space. Keep tightly closed.
After mixing, store suspension at 5° to 30°C (41° to 86°F) and use within 10 days. Discard after full dosing is completed.
-
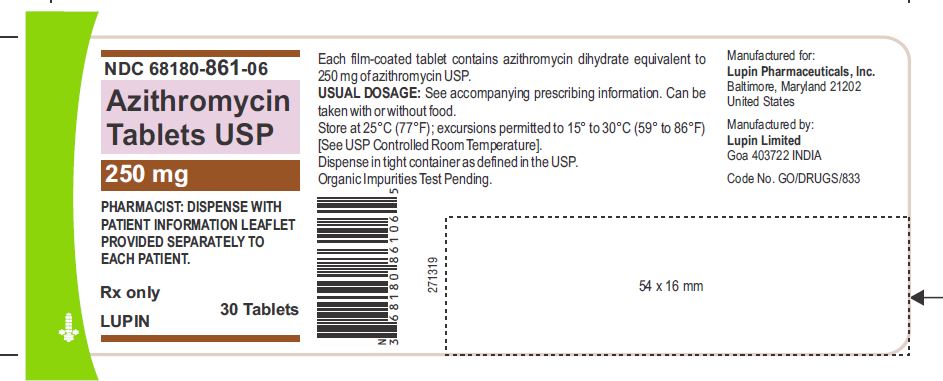
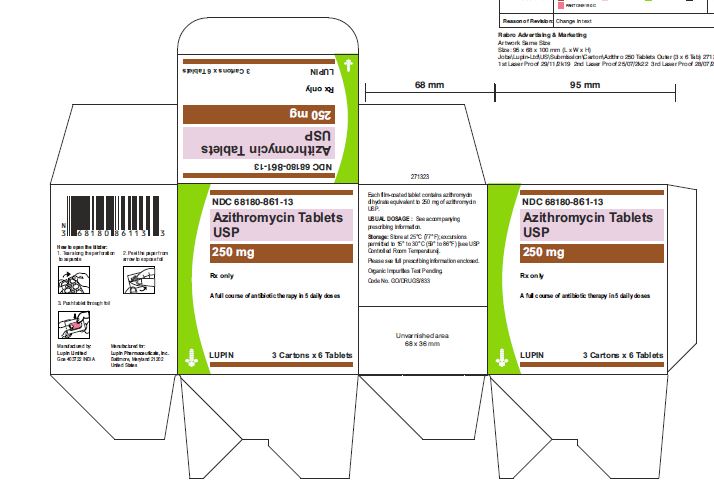
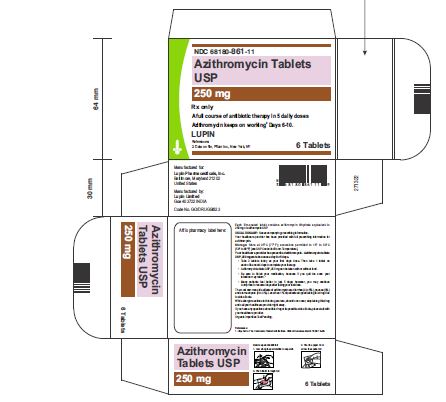
3 DOSAGE FORMS AND STRENGTHSAzithromycin Tablets, 250 mg are supplied as pink, oval shaped film-coated tablets, engraved with "LU" on one side and "L04" on the other side containing azithromycin dihydrate equivalent to ...Close
Azithromycin Tablets, 250 mg are supplied as pink, oval shaped film-coated tablets, engraved with "LU" on one side and "L04" on the other side containing azithromycin dihydrate equivalent to 250 mg of azithromycin USP.
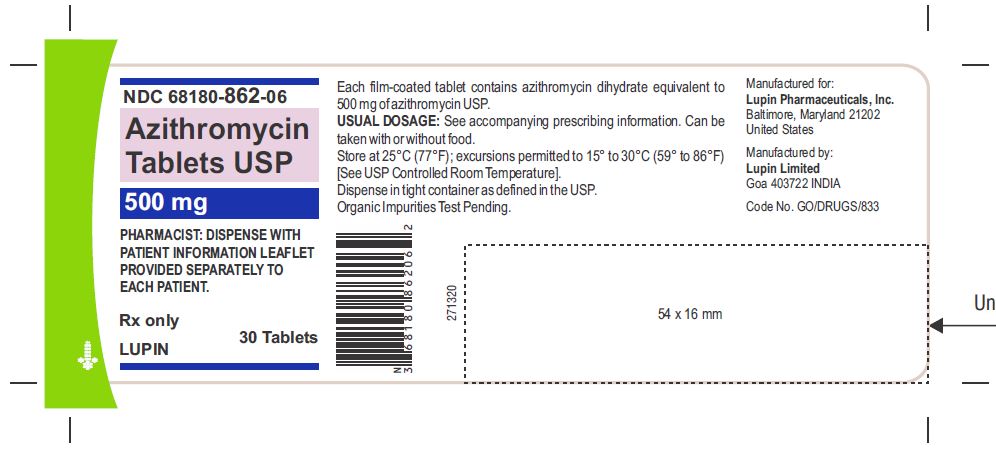
Azithromycin Tablets, 500 mg are supplied as pink, oval shaped film-coated tablets, engraved with "LU" on one side and "L05" on the other side containing azithromycin dihydrate equivalent to 500 mg of azithromycin USP.
-
4 CONTRAINDICATIONS4.1 Hypersensitivity - Azithromycin is contraindicated in patients with known hypersensitivity to azithromycin, erythromycin, any macrolide or ketolide drug. 4.2 Hepatic ...
4.1 Hypersensitivity
Azithromycin is contraindicated in patients with known hypersensitivity to azithromycin, erythromycin, any macrolide or ketolide drug.
Close4.2 Hepatic Dysfunction
Azithromycin is contraindicated in patients with a history of cholestatic jaundice/hepatic dysfunction associated with prior use of azithromycin.
-
5 WARNINGS AND PRECAUTIONS5.1 Hypersensitivity - Serious allergic reactions, including angioedema, anaphylaxis, and dermatologic reactions including Acute Generalized Exanthematous Pustulosis (AGEP), Stevens-Johnson ...
5.1 Hypersensitivity
Serious allergic reactions, including angioedema, anaphylaxis, and dermatologic reactions including Acute Generalized Exanthematous Pustulosis (AGEP), Stevens-Johnson syndrome, and toxic epidermal necrolysis have been reported in patients on azithromycin therapy. [see CONTRAINDICATIONS (4.1)]
Fatalities have been reported. Cases of Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) have also been reported. Despite initially successful symptomatic treatment of the allergic symptoms, when symptomatic therapy was discontinued, the allergic symptoms recurred soon thereafter in some patients without further azithromycin exposure. These patients required prolonged periods of observation and symptomatic treatment. The relationship of these episodes to the long tissue half-life of azithromycin and subsequent prolonged exposure to antigen is presently unknown.
If an allergic reaction occurs, the drug should be discontinued and appropriate therapy should be instituted. Physicians should be aware that allergic symptoms may reappear when symptomatic therapy has been discontinued.
5.2 Hepatotoxicity
Abnormal liver function, hepatitis, cholestatic jaundice, hepatic necrosis, and hepatic failure have been reported, some of which have resulted in death. Discontinue azithromycin immediately if signs and symptoms of hepatitis occur.
5.3 Infantile Hypertrophic Pyloric Stenosis (IHPS)
Following the use of azithromycin in neonates (treatment up to 42 days of life), IHPS has been reported. Direct parents and caregivers to contact their physician if vomiting or irritability with feeding occurs.
5.4 QT Prolongation
Prolonged cardiac repolarization and QT interval, imparting a risk of developing cardiac arrhythmia and torsades de pointes, have been seen with treatment with macrolides, including azithromycin. Cases of torsades de pointes have been spontaneously reported during postmarketing surveillance in patients receiving azithromycin. Providers should consider the risk of QT prolongation which can be fatal when weighing the risks and benefits of azithromycin for at-risk groups including:
- patients with known prolongation of the QT interval, a history of torsades de pointes, congenital long QT syndrome, bradyarrhythmias or uncompensated heart failure
- patients on drugs known to prolong the QT interval
- patients with ongoing proarrhythmic conditions such as uncorrected hypokalemia or hypomagnesemia, clinically significant bradycardia, and in patients receiving Class IA (quinidine, procainamide) or Class III (dofetilide, amiodarone, sotalol) antiarrhythmic agents.
Elderly patients may be more susceptible to drug-associated effects on the QT interval.
5.5 Cardiovascular Death
Some observational studies have shown an approximately two-fold increased short-term potential risk of acute cardiovascular death in adults exposed to azithromycin relative to other antibacterial drugs, including amoxicillin. The five-day cardiovascular mortality observed in these studies ranged from 20 to 400 per million azithromycin treatment courses. This potential risk was noted to be greater during the first five days of azithromycin use and does not appear to be limited to those patients with preexisting cardiovascular diseases. The data in these observational studies are insufficient to establish or exclude a causal relationship between acute cardiovascular death and azithromycin use. Consider balancing this potential risk with treatment benefits when prescribing Azithromycin tablets.
5.6 Clostridioides difficile-Associated Diarrhea
Clostridioides difficile-associated diarrhea has been reported with use of nearly all antibacterial agents, including azithromycin, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon, leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antibacterial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
5.7 Exacerbation of Myasthenia Gravis
Exacerbation of symptoms of myasthenia gravis and new onset of myasthenic syndrome have been reported in patients receiving azithromycin therapy.
5.8 Use in Sexually Transmitted Disease
Azithromycin, at the recommended dose, should not be relied upon to treat syphilis. Antibacterial agents used to treat non-gonococcal urethritis may mask or delay the symptoms of incubating syphilis. All patients with sexually transmitted urethritis or cervicitis should have a serologic test for syphilis and appropriate testing for gonorrhea performed at the time of diagnosis. Appropriate antibacterial therapy and follow-up tests for these diseases should be initiated if infection is confirmed.
Close5.9 Development of Drug-Resistant Bacteria
Prescribing azithromycin in the absence of a proven or strongly suspected bacterial infection is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in labeling: Hypersensitivity [see WARNINGS AND PRECAUTIONS (5.1)] Hepatotoxicity [see WARNINGS AND PRECAUTIONS ...
The following clinically significant adverse reactions are described elsewhere in labeling:
Hypersensitivity [see WARNINGS AND PRECAUTIONS (5.1)]
Hepatotoxicity [see WARNINGS AND PRECAUTIONS (5.2)]
Infantile Hypertrophic Pyloric Stenosis (IHPS) [see WARNINGS AND PRECAUTIONS (5.3)]
QT Prolongation [see WARNINGS AND PRECAUTIONS (5.4)]
Cardiovascular Death [see WARNINGS AND PRECAUTIONS (5.5)]
Clostridioides difficile-Associated Diarrhea (CDAD) [see WARNINGS AND PRECAUTIONS (5.6)]
Exacerbation of Myasthenia Gravis [see WARNINGS AND PRECAUTIONS (5.7)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In clinical trials, most of the reported side effects were mild to moderate in severity and were reversible upon discontinuation of the drug. Potentially serious adverse reactions of angioedema and cholestatic jaundice were reported. Approximately 0.7% of the patients (adults and pediatric patients) from the 5-day multiple-dose clinical trials discontinued azithromycin therapy because of treatment-related adverse reactions. In adults given 500 mg/day for 3 days, the discontinuation rate due to treatment-related adverse reactions was 0.6%. In clinical trials in pediatric patients given 30 mg/kg, either as a single dose or over 3 days, discontinuation from the trials due to treatment-related adverse reactions was approximately 1%. Most of the adverse reactions leading to discontinuation were related to the gastrointestinal tract, e.g., nausea, vomiting, diarrhea, or abdominal pain. [see CLINICAL STUDIES (14.2)]
Adults
Multiple-dose regimens: Overall, the most common treatment-related adverse reactions in adult patients receiving multiple-dose regimens of azithromycin were related to the gastrointestinal system with diarrhea/loose stools (4 to 5%), nausea (3%), and abdominal pain (2 to 3%) being the most frequently reported.
No other adverse reactions occurred in patients on the multiple-dose regimens of azithromycin with a frequency greater than 1%. Adverse reactions that occurred with a frequency of 1% or less included the following:
Cardiovascular:
Palpitations, chest pain.
Gastrointestinal:
Dyspepsia, flatulence, vomiting, melena, and cholestatic jaundice.
Genitourinary:
Monilia, vaginitis, and nephritis.
Nervous System:
Dizziness, headache, vertigo, and somnolence.
General:
Fatigue.
Allergic:
Rash, pruritus, photosensitivity, and angioedema.
Single 1-gram dose regimen:
Overall, the most common adverse reactions in patients receiving a single-dose regimen of 1 gram of azithromycin were related to the gastrointestinal system and were more frequently reported than in patients receiving the multiple-dose regimen.
Adverse reactions that occurred in patients on the single 1-gram dosing regimen of azithromycin with a frequency of 1% or greater included diarrhea/loose stools (7%), nausea (5%), abdominal pain (5%), vomiting (2%), dyspepsia (1%), and vaginitis (1%).
Single 2-gram dose regimen:
Overall, the most common adverse reactions in patients receiving a single 2-gram dose of azithromycin were related to the gastrointestinal system. Adverse reactions that occurred in patients in this study with a frequency of 1% or greater included nausea (18%), diarrhea/loose stools (14%), vomiting (7%), abdominal pain (7%), vaginitis (2%), dyspepsia (1%), and dizziness (1%). The majority of these complaints were mild in nature.
Pediatric Patients
Single and Multiple-dose regimens: The types of adverse reactions in pediatric patients were comparable to those seen in adults, with different incidence rates for the dosage regimens recommended in pediatric patients.
Acute Otitis Media:
For the recommended total dosage regimen of 30 mg/kg, the most frequent adverse reactions (≥1%) attributed to treatment were diarrhea, abdominal pain, vomiting, nausea, and rash. [see DOSAGE AND ADMINISTRATION (2) and CLINICAL STUDIES (14.2)]
The incidence, based on dosing regimen, is described in the table below:
Dosage Regimen
Diarrhea %
Abdominal Pain %
Vomiting %
Nausea %
Rash %
1-day
4.3%
1.4%
4.9%
1.0%
1.0%
3-day
2.6%
1.7%
2.3%
0.4%
0.6%
5-day
1.8%
1.2%
1.1%
0.5%
0.4%
For the recommended dosage regimen of 10 mg/kg on Day 1 followed by 5 mg/kg on Days 2 to 5, the most frequent adverse reactions attributed to treatment were diarrhea/loose stools, abdominal pain, vomiting, nausea, and rash.
The incidence is described in the table below:
Dosage Regimen
Diarrhea/Loose stools %
Abdominal Pain %
Vomiting %
Nausea %
Rash %
5-day
5.8%
1.9%
1.9%
1.9%
1.6%
For the recommended dosage regimen of 12 mg/kg on Days 1 to 5, the most frequent adverse reactions attributed to treatment were diarrhea, vomiting, abdominal pain, nausea, and headache.
The incidence is described in the table below:
Dosage Regimen
Diarrhea %
Abdominal Pain %
Vomiting %
Nausea %
Rash %
Headache %
5-day
5.4%
3.4%
5.6%
1.8%
0.7%
1.1%
With any of the treatment regimens, no other adverse reactions occurred in pediatric patients treated with azithromycin with a frequency greater than 1%. Adverse reactions that occurred with a frequency of 1% or less included the following:
Cardiovascular:
Chest pain.
Gastrointestinal:
Dyspepsia, constipation, anorexia, enteritis, flatulence, gastritis, jaundice, loose stools, and oral moniliasis.
Hematologic and Lymphatic:
Anemia and leukopenia.
Nervous System:
Headache (otitis media dosage), hyperkinesia, dizziness, agitation, nervousness, and insomnia.
General:
Fever, face edema, fatigue, fungal infection, malaise, and pain.
Allergic:
Rash and allergic reaction.
Respiratory:
Cough, pharyngitis, pleural effusion, and rhinitis.
Skin and Appendages:
Eczema, fungal dermatitis, pruritus, sweating, urticaria, and vesiculobullous rash.
Special Senses:
Conjunctivitis.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of azithromycin. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Adverse reactions reported with azithromycin during the postmarketing period in adult and/or pediatric patients for which a causal relationship may not be established include:
Allergic:
Arthralgia, edema, urticaria, and angioedema.
Cardiovascular:
Arrhythmias including ventricular tachycardia and hypotension. There have been reports of QT prolongation, torsades de pointes and cardiovascular death.
Gastrointestinal:
Anorexia, constipation, dyspepsia, flatulence, vomiting/diarrhea, pseudomembranous colitis, pancreatitis, oral candidiasis, pyloric stenosis, and reports of tongue discoloration.
General:
Asthenia, paresthesia, fatigue, malaise, and anaphylaxis.
Genitourinary:
Interstitial nephritis and acute renal failure and vaginitis.
Hematopoietic:
Thrombocytopenia.
Liver/Biliary:
Abnormal liver function, hepatitis, cholestatic jaundice, hepatic necrosis, and hepatic failure. [see WARNINGS AND PRECAUTIONS (5.2)]
Nervous System:
Convulsions, dizziness/vertigo, headache, somnolence, hyperactivity, nervousness, agitation, and syncope.
Psychiatric:
Aggressive reaction and anxiety.
Skin/Appendages:
Pruritus serious skin reactions including erythema multiforme, AGEP, Stevens-Johnson Syndrome, toxic epidermal necrolysis, and DRESS
Special Senses:
Hearing disturbances including hearing loss, deafness and/or tinnitus, and reports of taste/smell perversion and/or loss.
Close6.3 Laboratory Abnormalities
Clinically significant abnormalities (irrespective of drug relationship) occurring during the clinical trials were reported as follows: with an incidence of greater than 1%: decreased hemoglobin, hematocrit, lymphocytes, neutrophils, and blood glucose; elevated serum creatine phosphokinase, potassium, ALT, GGT, AST, BUN, creatinine, blood glucose, platelet count, lymphocytes, neutrophils, and eosinophils; with an incidence of less than 1%: leukopenia, neutropenia, decreased sodium, potassium, platelet count, elevated monocytes, basophils, bicarbonate, serum alkaline phosphatase, bilirubin, LDH, and phosphate. The majority of subjects with elevated serum creatinine also had abnormal values at baseline. When follow-up was provided, changes in laboratory tests appeared to be reversible.
In multiple-dose clinical trials involving more than 5000 patients, four patients discontinued therapy because of treatment-related liver enzyme abnormalities and one because of a renal function abnormality.
Pediatric Patients
One, Three, and Five Day Regimens:
Laboratory data collected from comparative clinical trials employing two 3-day regimens (30 mg/kg or 60 mg/kg in divided doses over 3 days), or two 5-day regimens (30 mg/kg or 60 mg/kg in divided doses over 5 days) were similar for regimens of azithromycin and all comparators combined, with most clinically significant laboratory abnormalities occurring at incidences of 1 to 5%. Laboratory data for patients receiving 30 mg/kg as a single dose were collected in one single center trial. In that trial, an absolute neutrophil count between 500 to 1500 cells/mm3was observed in 10/64 patients receiving 30 mg/kg as a single dose, 9/62 patients receiving 30 mg/kg given over 3 days, and 8/63 comparator patients. No patient had an absolute neutrophil count <500 cells/mm3.
In multiple-dose clinical trials involving approximately 4700 pediatric patients, no patients discontinued therapy because of treatment-related laboratory abnormalities.
-
7 DRUG INTERACTIONS7.1 Nelfinavir - Co-administration of nelfinavir at steady-state with a single oral dose of azithromycin resulted in increased azithromycin serum concentrations. Although a dose adjustment of ...
7.1 Nelfinavir
Co-administration of nelfinavir at steady-state with a single oral dose of azithromycin resulted in increased azithromycin serum concentrations. Although a dose adjustment of azithromycin is not recommended when administered in combination with nelfinavir, close monitoring for known adverse reactions of azithromycin, such as liver enzyme abnormalities and hearing impairment, is warranted. [see ADVERSE REACTIONS (6)]
7.2 Warfarin
Spontaneous postmarketing reports suggest that concomitant administration of azithromycin may potentiate the effects of oral anticoagulants such as warfarin, although the prothrombin time was not affected in the dedicated drug interaction study with azithromycin and warfarin. Prothrombin times should be carefully monitored while patients are receiving azithromycin and oral anticoagulants concomitantly.
Close7.3 Potential Drug-Drug Interaction with Macrolides
Interactions with digoxin, colchicine or phenytoin have not been reported in clinical trials with azithromycin. No specific drug interaction studies have been performed to evaluate potential drug-drug interaction. However, drug interactions have been observed with other macrolide products. Until further data are developed regarding drug interactions when digoxin, colchicine or phenytoin are used with azithromycin careful monitoring of patients is advised.
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available data from published literature and postmarketing experience over several decades with azithromycin use in pregnant women have not identified any ...
8.1 Pregnancy
Available data from published literature and postmarketing experience over several decades with azithromycin use in pregnant women have not identified any drug-associated risks for major birth defects, miscarriage, or adverse maternal or fetal outcomes (see Data). Developmental toxicity studies with azithromycin in rats, mice, and rabbits showed no drug-induced fetal malformations at doses up to 4, 2, and 2 times, respectively, an adult human daily dose of 500 mg based on body surface area. Decreased viability and delayed development were observed in the offspring of pregnant rats administered azithromycin from day 6 of pregnancy through weaning at a dose equivalent to 4 times an adult human daily dose of 500 mg based on body surface area (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated populations is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Human Data:
Available data from published observational studies, case series, and case reports over several decades do not suggest an increased risk for major birth defects, miscarriage, or adverse maternal or fetal outcomes with azithromycin use in pregnant women. Limitations of these data include the lack of randomization and inability to control for confounders such as underlying maternal disease and maternal use of concomitant medications.
Animal Data:
Azithromycin administered during the period of organogenesis did not cause fetal malformations in rats and mice at oral doses up to 200 mg/kg/day (moderately maternally toxic). Based on body surface area, this dose is approximately 4 (rats) and 2 (mice) times an adult human daily dose of 500 mg. In rabbits administered azithromycin at oral doses of 10, 20, and 40 mg/kg/day during organogenesis, reduced maternal body weight and food consumption were observed in all groups; no evidence of fetotoxicity or teratogenicity was observed at these doses, the highest of which is estimated to be 2 times an adult human daily dose of 500 mg based on body surface area.
In a pre-and postnatal development study, azithromycin was administered orally to pregnant rats from day 6 of pregnancy until weaning at doses of 50 or 200 mg/kg/day. Maternal toxicity (reduced food consumption and body weight gain; increased stress at parturition) was observed at the higher dose. Effects in the offspring were noted at 200 mg/kg/day during the postnatal development period (decreased viability, delayed developmental landmarks). These effects were not observed in a pre-and postnatal rat study when up to 200 mg/kg/day of azithromycin was given orally beginning on day 15 of pregnancy until weaning.
8.2 Lactation
Azithromycin is present in human milk (see Data). Non-serious adverse reactions have been reported in breastfed infants after maternal administration of azithromycin (see Clinical Considerations). There are no available data on the effects of azithromycin on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for azithromycin and any potential adverse effects on the breastfed infant from azithromycin or from the underlying maternal condition.
Clinical Considerations
Advise women to monitor the breastfed infant for diarrhea, vomiting, or rash.
Data
Azithromycin breastmilk concentrations were measured in 20 women after receiving a single 2 g oral dose of azithromycin during labor. Breastmilk samples collected on days 3 and 6 postpartum as well as 2 and 4 weeks postpartum revealed the presence of azithromycin in breastmilk up to 4 weeks after dosing. In another study, a single dose of azithromycin 500 mg was administered intravenously to 8 women prior to incision for cesarean section. Breastmilk (colostrum) samples obtained between 12 and 48 hours after dosing revealed that azithromycin persisted in breastmilk up to 48 hours.
8.4 Pediatric Use
[see CLINICAL PHARMACOLOGY (12.3), INDICATIONS AND USAGE (1.2), and DOSAGE AND ADMINISTRATION (2.2)]
Safety and effectiveness in the treatment of pediatric patients with acute otitis media, acute bacterial sinusitis and community-acquired pneumonia under 6 months of age have not been established. Use of azithromycin for the treatment of acute bacterial sinusitis and community-acquired pneumonia in pediatric patients (6 months of age or greater) is supported by adequate and well-controlled trials in adults.
Pharyngitis/Tonsillitis
Safety and effectiveness in the treatment of pediatric patients with pharyngitis/tonsillitis under 2 years of age have not been established.
Close8.5 Geriatric Use
In multiple-dose clinical trials of oral azithromycin, 9% of patients were at least 65 years of age (458/4949) and 3% of patients (144/4949) were at least 75 years of age. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in response between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Elderly patients may be more susceptible to development of torsades de pointes arrhythmias than younger patients. [see WARNINGS AND PRECAUTIONS (5.4)]
-
10 OVERDOSAGEAdverse reactions experienced at higher than recommended doses were similar to those seen at normal doses particularly nausea, diarrhea, and vomiting. In the event of overdosage, general ...
Adverse reactions experienced at higher than recommended doses were similar to those seen at normal doses particularly nausea, diarrhea, and vomiting. In the event of overdosage, general symptomatic and supportive measures are indicated as required.
Close -
11 DESCRIPTIONAzithromycin tablets USP contain the active ingredient azithromycin, a macrolide antibacterial drug, for oral administration. Azithromycin has the chemical name ...
Azithromycin tablets USP contain the active ingredient azithromycin, a macrolide antibacterial drug, for oral administration. Azithromycin has the chemical name (2R,3S,4R,5R,8R,10R,11R,12S,13S,14R)-13-[(2,6-dideoxy-3-C-methyl-3-O-methyl-α-L-ribo-hexopyranosyl) oxy]-2-ethyl-3,4,10-trihydroxy-3,5,6,8,10,12,14-heptamethyl-11-[[3,4,6-trideoxy-3-(dimethylamino)-β-D-xylo-hexopyranosyl]oxy]-1-oxa-6-azacyclopentadecan-15-one. Azithromycin is derived from erythromycin; however, it differs chemically from erythromycin in that a methyl-substituted nitrogen atom is incorporated into the lactone ring. Its molecular formula is C38H72N2O12, and its molecular weight is 749.00. Azithromycin has the following structural formula:
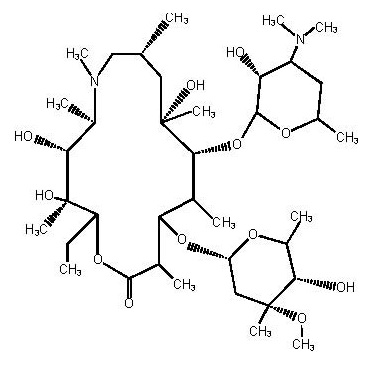
Azithromycin, as the dihydrate, is a white to almost white crystalline powder with a molecular formula of C38H72N2O12•2H2O and a molecular weight of 785.02.
Azithromycin is supplied as tablets containing azithromycin dihydrate equivalent to either 250 mg or 500 mg azithromycin and the following inactive ingredients: croscarmellose sodium, dibasic calcium phosphate, hydroxypropyl methyl cellulose, lactose monohydrate, magnesium stearate, sodium lauryl sulfate, titanium dioxide, triacetin and D&C Red #30.
Organic Impurities Test Pending.
Close -
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Azithromycin is a macrolide antibacterial drug. [see Microbiology (12.4)] 12.2 Pharmacodynamics - Based on animal models of infection, the antibacterial activity ...
12.2 Pharmacodynamics
Based on animal models of infection, the antibacterial activity of azithromycin appears to correlate with the ratio of area under the concentration-time curve to minimum inhibitory concentration (AUC/MIC) for certain pathogens (S. pneumoniae and S. aureus). The principal pharmacokinetic/pharmacodynamic parameter best associated with clinical and microbiological cure has not been elucidated in clinical trials with azithromycin.
Cardiac Electrophysiology
QTc interval prolongation was studied in a randomized, placebo-controlled parallel trial in 116 healthy subjects who received either chloroquine (1000 mg) alone or in combination with oral azithromycin (500 mg, 1000 mg, and 1500 mg once daily). Co-administration of azithromycin increased the QTc interval in a dose- and concentration- dependent manner. In comparison to chloroquine alone, the maximum mean (95% upper confidence bound) increases in QTcF were 5 (10) ms, 7 (12) ms and 9 (14) ms with the co-administration of 500 mg, 1000 mg and 1500 mg azithromycin, respectively.
12.3 Pharmacokinetics
Following oral administration of a single 500 mg dose (two 250 mg tablets) to 36 fasted healthy male volunteers, the mean (SD) pharmacokinetic parameters were AUC0-72=4.3 (1.2) mcg·hr/mL; Cmax=0.5 (0.2) mcg/mL; Tmax =2.2 (0.9) hours. Two azithromycin 250 mg tablets are bioequivalent to a single 500 mg tablet.
In a two-way crossover study, 12 adult healthy volunteers (6 males, 6 females) received 1500 mg of azithromycin administered in single daily doses over either 5 days (two 250 mg tablets on day 1, followed by one 250 mg tablet on days 2 to 5) or 3 days (500 mg per day for days 1 to 3). Due to limited serum samples on day 2 (3-day regimen) and days 2 to 4 (5-day regimen), the serum concentration-time profile of each subject was fit to a 3-compartment model and the AUC 0-∞ for the fitted concentration profile was comparable between the 5-day and 3-day regimens.
*Total AUC for the entire 3-day and 5-day regimens.
3-Day Regimen
5-Day Regimen
Pharmacokinetic Parameter [mean (SD)]
Day 1
Day 3
Day 1
Day 5
Cmax (serum, mcg/mL)
0.44 (0.22)
0.54 (0.25)
0.43 (0.20)
0.24 (0.06)
Serum AUC 0-∞ (mcg·hr/mL)
17.4 (6.2)*
14.9 (3.1)*
Serum T 1/2
71.8 hr
68.9 hr
The absolute bioavailability of azithromycin 250 mg capsules is 38%.
In a two-way crossover study in which 12 healthy subjects received a single 500 mg dose of azithromycin (two 250 mg tablets) with or without a high fat meal, food was shown to increase
Cmax by 23% but had no effect on AUC.
When azithromycin oral suspension was administered with food to 28 adult healthy male subjects, Cmax increased by 56% and AUC was unchanged.
Distribution
The serum protein binding of azithromycin is variable in the concentration range approximating human exposure, decreasing from 51% at 0.02 mcg/mL to 7% at 2 mcg/mL.
The antibacterial activity of azithromycin is pH related and appears to be reduced with decreasing pH, However, the extensive distribution of drug to tissues may be relevant to clinical activity.
Azithromycin has been shown to penetrate into human tissues, including skin, lung, tonsil, and cervix. Extensive tissue distribution was confirmed by examination of additional tissues and fluids (bone, ejaculum, prostate, ovary, uterus, salpinx, stomach, liver, and gallbladder). As there are no data from adequate and well-controlled studies of azithromycin treatment of infections in these additional body sites, the clinical significance of these tissue concentration data is unknown.
Following a regimen of 500 mg on the first day and 250 mg daily for 4 days, very low concentrations were noted in cerebrospinal fluid (less than 0.01 mcg/mL) in the presence of noninflamed meninges.
Metabolism
In vitro and in vivo studies to assess the metabolism of azithromycin have not been performed.
Elimination
Plasma concentrations of azithromycin following single 500 mg oral and IV doses declined in a polyphasic pattern resulting in a mean apparent plasma clearance of 630 mL/min and terminal elimination half-life of 68hr. The prolonged terminal half-life is thought to be due to extensive uptake and subsequent release of drug from tissues. Biliary excretion of azithromycin, predominantly as unchanged drug, is a major route of elimination. Over the course of a week, approximately 6% of the administered dose appears as unchanged drug in urine.
Specific Populations
Patients with Renal Impairment:
Azithromycin pharmacokinetics was investigated in 42 adults (21 to 85 years of age) with varying degrees of renal impairment. Following the oral administration of a single 1.0 g dose of azithromycin (4 x 250 mg capsules), mean Cmax and AUC0-120 increased by 5.1% and 4.2%, respectively, in subjects with mild to moderate renal impairment (GFR 10 to 80 mL/min) compared to subjects with normal renal function (GFR >80 mL/min). The mean Cmax and AUC0-120 increased 61% and 35%, respectively, in subjects with severe renal impairment (GFR <10 mL/min) compared to subjects with normal renal function (GFR >80 mL/min).
Patients with Hepatic Impairment:
The pharmacokinetics of azithromycin in subjects with hepatic impairment has not been established.
Male and Female Patients:
There are no significant differences in the disposition of azithromycin between male and female subjects. No dosage adjustment is recommended based on gender.
Geriatric Patients:
Pharmacokinetic parameters in older volunteers (65 to 85 years old) were similar to those in young adults (18 to 40 years old) for the 5-day therapeutic regimen. Dosage adjustment does not appear to be necessary for older patients with normal renal and hepatic function receiving treatment with this dosage regimen. [see Geriatric Use (8.5)]
Pediatric Patients:
In two clinical studies, azithromycin for oral suspension was dosed at 10 mg/kg on day 1, followed by 5 mg/kg on days 2 through 5 in two groups of pediatric patients (aged 1 to 5 years and 5 to 15 years, respectively). The mean pharmacokinetic parameters on day 5 were Cmax =0.216 mcg/mL, Tmax =1.9 hr, and AUC0-24=1.822 mcg·hr/mL for the 1 to 5-year-old group and were Cmax =0.383 mcg/mL, Tmax =2.4 hr, and AUC0-24 =3.109 mcg·hr/mL for the 5 to 15-year-old group.
In another study, 33 pediatric patients received doses of 12 mg/kg/day (maximum daily dose 500 mg) for 5 days, of whom 31 patients were evaluated for azithromycin pharmacokinetics following a low fat breakfast. In this study, azithromycin concentrations were determined over a 24 hr period following the last daily dose. Patients weighing above 41.7 kg received the maximum adult daily dose of 500 mg. Seventeen patients (weighing 41.7 kg or less) received a total dose of 60 mg/kg. The following table shows pharmacokinetic data in the subset of pediatric patients who received a total dose of 60 mg/kg.
Pharmacokinetic Parameter
[mean (SD)]
5-Day Regimen
(12 mg/kg for 5 days)
N
17
Cmax (mcg/mL)
0.5 (0.4)
Tmax (hr)
2.2 (0.8)
AUC 0-24(mcg⋅hr/mL)
3.9 (1.9)
Single dose pharmacokinetics of azithromycin in pediatric patients given doses of 30 mg/kg have not been studied. [see DOSAGE AND ADMINISTRATION (2)]
Drug Interaction Studies
Drug interaction studies were performed with azithromycin and other drugs likely to be co-administered. The effects of co -administration of azithromycin on the pharmacokinetics of other drugs are shown in Table 1 and the effects of other drugs on the pharmacokinetics of azithromycin are shown in Table 2.
Co-administration of azithromycin at therapeutic doses had a modest effect on the pharmacokinetics of the drugs listed in Table 1. No dosage adjustment of drugs listed in Table 1 is recommended when co-administered with azithromycin.
Co-administration of azithromycin with efavirenz or fluconazole had a modest effect on the pharmacokinetics of azithromycin. Nelfinavir significantly increased the Cmax and AUC of azithromycin. No dosage adjustment of azithromycin is recommended when administered with drugs listed in Table 2. [see DRUG INTERACTIONS (7.3)]
* -90% Confidence interval not reported
Table 1. Drug Interactions: Pharmacokinetic Parameters for Co-administered Drugs in the Presence of Azithromycin
Co-administered Drug
Dose of Co-administered Drug
Dose of Azithromycin
n
Ratio (with/without azithromycin) of
Co-administered Drug Pharmacokinetic Parameters (90% CI); No Effect = 1.00
Mean Cmax
Mean AUC
Atorvastatin
10 mg/day for 8 days
500 mg/day orally on days 6 to 8
12
0.83
(0.63 to 1.08)
1.01
(0.81 to 1.25)
Carbamazepine
200 mg/day for 2 days, then 200 mg twice a day for 18 days
500 mg/day orally for days 16 to 18
7
0.97
(0.88 to 1.06)
0.96
(0.88 to 1.06)
Cetirizine
20 mg/day for 11 days
500 mg orally on day 7, then 250 mg/day on days 8 to 11
14
1.03
(0.93 to 1.14)
1.02
(0.92 to 1.13)
Didanosine
200 mg orally twice a day for 21 days
1200 mg/day orally on days 8 to 21
6
1.44
(0.85 to 2.43)
1.14
(0.83 to 1.57)
Efavirenz
400 mg/day for 7 days
600 mg orally on day 7
14
1.04*
0.95*
Fluconazole
200 mg orally single dose
1200 mg orally single dose
18
1.04
(0.98 to 1.11)
1.01
(0.97 to 1.05)
Indinavir
800 mg three times a day for 5 days
1200 mg orally on day 5
18
0.96
(0.86 to 1.08)
0.90
(0.81 to 1.00)
Midazolam
15 mg orally on day 3
500 mg/day orally for 3 days
12
1.27
(0.89 to 1.81)
1.26
(1.01 to 1.56)
Nelfinavir
750 mg three times a day for 11 days
1,200 mg orally on day 9
14
0.90
(0.81 to 1.01)
0.85
(0.78 to 0.93)
Sildenafil
100 mg on days 1 and 4
500 mg/day orally for 3 days
12
1.16
(0.86 to 1.57)
0.92
(0.75 to 1.12)
Theophylline
4 mg/kg IV on days 1, 11, 25
500 mg orally on day 7, 250 mg/day on days 8 to 11
10
1.19
(1.02 to 1.40)
1.02
(0.86 to 1.22)
Theophylline
300 mg orally twice a day for 15 days
500 mg orally on day 6, then 250 mg/day on days 7 to 10
8
1.09
(0.92 to 1.29)
1.08
(0.89 to 1.31)
Triazolam
0.125 mg on day 2
500 mg orally on day 1, then 250 mg/day on day 2
12
1.06*
1.02*
Trimethoprim/ Sulfamethoxazole
160 mg/800 mg/day orally for 7 days
1200 mg orally on day 7
12
0.85
(0.75 to 0.97)/0.90 (0.78 to 1.03)
0.87 (0.80 to 0.95/0.96
(0.88 to 1.03)
Zidovudine
500 mg/day orally for 21 days
600 mg/day orally for 14 days
5
1.12
(0.42 to 3.02)
0.94
(0.52 to 1.70)
Zidovudine
500 mg/day orally for 21 days
1200 mg/day orally for 14 days
4
1.31
(0.43 to 3.97)
1.30
(0.69 to 2.43)
* -90% Confidence interval not reported
Table 2. Drug Interactions: Pharmacokinetic Parameters for Azithromycin in the Presence of Co-administered Drugs. [see DRUG INTERACTIONS (7)] Co-administered Drug
Dose of Co-administered Drug
Dose of Azithromycin
n
Ratio (with/without co-administered drug) of Azithromycin Pharmacokinetic Parameters
(90% CI); No Effect = 1.00
Mean Cmax
Mean AUC
Efavirenz
400 mg/day for 7 days
600 mg orally on day 7
14
1.22
(1.04 to 1.42)
0.92*
Fluconazole
200 mg orally single dose
1,200 mg orally single dose
18
0.82
(0.66 to 1.02)
1.07
(0.94 to 1.22)
Nelfinavir
750 mg three times a day for 11 days
1,200 mg orally on day 9
14
2.36
(1.77 to 3.15)
2.12
(1.80 to 2.50)
Close12.4 Microbiology
Azithromycin acts by binding to the 23S rRNA of the 50S ribosomal subunit of susceptible microorganisms inhibiting bacterial protein synthesis and impeding the assembly of the 50S ribosomal subunit.
Resistance
Azithromycin demonstrates cross resistance with erythromycin. The most frequently encountered mechanism of resistance to azithromycin is modification of the 23S rRNA target, most often by methylation. Ribosomal modifications can determine cross resistance to other macrolides, lincosamides, and streptogramin B (MLSB phenotype).
Antimicrobial Activity
Azithromycin has been shown to be active against most isolates of the following microorganisms, both in vitro and in clinical infections. [see INDICATIONS AND USAGE (1)]
Gram-Positive Bacteria
Staphylococcus aureus
Streptococcus agalactiae
Streptococcus pneumoniae
Streptococcus pyogenes
Gram-Negative Bacteria
Haemophilus ducreyi
Haemophilus influenzae
Moraxella catarrhalis
Neisseria gonorrhoeae
Other Bacteria
Chlamydophila pneumoniae
Chlamydia trachomatis
Mycoplasma pneumoniae
The following in vitro data are available, but their clinical significance is unknown. At least 90 percent of the following bacteria exhibit an in vitro minimum inhibitory concentration (MIC) less than or equal to the susceptible breakpoint for azithromycin against isolates of similar genus or organism group. However, the efficacy of azithromycin in treating clinical infections caused by these bacteria has not been established in adequate and well-controlled clinical trials.
Gram-Positive Bacteria
Beta-hemolytic streptococci (Groups C, F, G)
Viridans group streptococci
Gram-Negative Bacteria
Bordetella pertussis
Legionella pneumophila
Anaerobic Bacteria
Prevotella bivia
Peptostreptococcus species
Other Bacteria
Ureaplasma urealyticum
Susceptibility Testing
For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: https://www.fda.gov/STIC.
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term studies in animals have not been performed to evaluate carcinogenic potential. Azithromycin has shown no mutagenic potential ...
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals have not been performed to evaluate carcinogenic potential. Azithromycin has shown no mutagenic potential in standard laboratory tests: mouse lymphoma assay, human lymphocyte clastogenic assay, and mouse bone marrow clastogenic assay. In fertility studies conducted in male and female rats, oral administration of azithromycin for 64 to 66 days (males) or 15 days (females) prior to and during cohabitation resulted in decreased pregnancy rate at 20 and 30 mg/kg/day when both males and females were treated with azithromycin. This minimal effect on pregnancy rate (approximately 12% reduction compared to concurrent controls) did not become more pronounced when the dose was increased from 20 to 30 mg/kg/day (approximately 0.4 to 0.6 times the adult daily dose of 500 mg based on body surface area) and it was not observed when only one animal in the mated pair was treated. There were no effects on any other reproductive parameters, and there were no effects on fertility at 10 mg/kg/day. The relevance of these findings to patients being treated with azithromycin at the doses and durations recommended in the prescribing information is uncertain.
Close13.2 Animal Toxicology and/or Pharmacology
Phospholipidosis (intracellular phospholipid accumulation) has been observed in some tissues of mice, rats, and dogs given multiple doses of azithromycin. It has been demonstrated in numerous organ systems (e.g., eye, dorsal root ganglia, liver, gallbladder, kidney, spleen, and/or pancreas) in dogs and rats treated with azithromycin at doses which, expressed on the basis of body surface area, are similar to or less than the highest recommended adult human dose. This effect has been shown to be reversible after cessation of azithromycin treatment. Based on the pharmacokinetic data, phospholipidosis has been seen in the rat (50 mg/kg/day dose) at the observed maximal plasma concentration of 1.3 mcg/mL (1.6 times the observed Cmax of 0.821 mcg/mL at the adult dose of 2 g). Similarly, it has been shown in the dog (10 mg/kg/day dose) at the observed maximal serum concentration of 1 mcg/mL (1.2 times the observed Cmax of 0.821 mcg/mL at the adult dose of 2 g). Phospholipidosis was also observed in neonatal rats dosed for 18 days at 30 mg/kg/day, which is less than the pediatric dose of 60 mg/kg based on the surface area. It was not observed in neonatal rats treated for 10 days at 40 mg/kg/day with mean maximal serum concentrations of 1.86 mcg/mL, approximately 1.5 times the Cmax of 1.27 mcg/mL at the pediatric dose. Phospholipidosis has been observed in neonatal dogs (10 mg/kg/day) at maximum mean whole blood concentrations of 3.54 mcg/mL, approximately 3 times the pediatric dose Cmax. The significance of these findings for animals and for humans is unknown.
-
14 CLINICAL STUDIES14.1 Adult Patients - Acute Bacterial Exacerbations of Chronic Bronchitis - In a randomized, double-blind controlled clinical trial of acute exacerbation of chronic bronchitis (AECB) ...
14.1 Adult Patients
Acute Bacterial Exacerbations of Chronic Bronchitis
In a randomized, double-blind controlled clinical trial of acute exacerbation of chronic bronchitis (AECB), azithromycin (500 mg once daily for 3 days) was compared with clarithromycin (500 mg twice daily for 10 days). The primary endpoint of this trial was the clinical cure rate at Days 21 to 24. For the 304 patients analyzed in the modified intent-to-treat analysis at the Days 21 to 24 visit, the clinical cure rate for 3 days of azithromycin was 85% (125/147) compared to 82% (129/157) for 10 days of clarithromycin.
The following outcomes were the clinical cure rates at the Days 21 to 24 visit for the bacteriologically evaluable patients by pathogen:
Pathogen
Azithromycin (3 Days)
Clarithromycin (10 Days)
S. pneumoniae
29/32 (91%)
21/27 (78%)
H. influenzae
12/14 (86%)
14/16 (88%)
M. catarrhalis
11/12 (92%)
12/15 (80%)
In a randomized, double-blind, double-dummy controlled clinical trial of acute bacterial sinusitis, azithromycin (500 mg once daily for 3 days) was compared with amoxicillin/clavulanate (500/125 mg three times a day for 10 days). Clinical response assessments were made at Day 10 and Day 28. The primary endpoint of this trial was prospectively defined as the clinical cure rate at Day 28. For the 594 patients analyzed in the modified intent to treat analysis at the Day 10 visit, the clinical cure rate for 3 days of azithromycin was 88% (268/303) compared to 85% (248/291) for 10 days of amoxicillin/clavulanate. For the 586 patients analyzed in the modified intent to treat analysis at the Day 28 visit, the clinical cure rate for 3 days of azithromycin was 71.5% (213/298) compared to 71.5% (206/288), with a 97.5% confidence interval of –8.4 to 8.3, for 10 days of amoxicillin/clavulanate.
In an open label, non-comparative study requiring baseline transantral sinus punctures, the following outcomes were the clinical success rates at the Day 7 and Day 28 visits for the modified intent to treat patients administered 500 mg of azithromycin once daily for 3 days with the following pathogens:
Clinical Success Rates of Azithromycin (500 mg per day for 3 Days) Pathogen
Day 7
Day 28
S. pneumoniae
23/26 (88%)
21/25 (84%)
H. influenzae
28/32 (87%)
24/32 (75%)
M. catarrhalis
14/15 (93%)
13/15 (87%)
Close14.2 Pediatric Patients
From the perspective of evaluating pediatric clinical trials, Days 11 to 14 were considered on-therapy evaluations because of the extended half-life of azithromycin. Days 11 to 14 data are provided for clinical guidance. Days 24 to 32 evaluations were considered the primary test of cure endpoint.
Pharyngitis/Tonsillitis
In three double-blind controlled studies, conducted in the United States, azithromycin (12 mg/kg once a day for 5 days) was compared to penicillin V (250 mg three times a day for 10 days) in the treatment of pharyngitis due to documented Group A β-hemolytic streptococci (GABHS or S. pyogenes). Azithromycin was clinically and microbiologically statistically superior to penicillin at Day 14 and Day 30 with the following clinical success (i.e., cure and improvement) and bacteriologic efficacy rates (for the combined evaluable patient with documented GABHS):
Three U.S. Streptococcal Pharyngitis Studies Azithromycin vs. Penicillin V EFFICACY RESULTS
Day 14
Day 30
Bacteriologic Eradication:
Azithromycin
323/340 (95%)
255/330 (77%)
Penicillin V
242/332 (73%)
206/325 (63%)
Clinical Success (cure plus improvement):
Azithromycin
336/343 (98%)
310/330 (94%)
Penicillin V
284/338 (84%)
241/325 (74%)
Approximately 1% of azithromycin-susceptible S. pyogenes isolates were resistant to azithromycin following therapy.
Acute Otitis Media
Efficacy using azithromycin given over 5 days (10 mg/kg on Day 1 followed by 5 mg/kg on Days 2 to 5):
Trial 1
In a double-blind, controlled clinical study of acute otitis media performed in the United States, azithromycin (10 mg/kg on Day 1 followed by 5 mg/kg on Days 2 to 5) was compared to amoxicillin/clavulanate potassium (4:1). For the 553 patients who were evaluated for clinical efficacy, the clinical success rate (i.e., cure plus improvement) at the Day 11 visit was 88% for azithromycin and 88% for the control agent. For the 521 patients who were evaluated at the Day 30 visit, the clinical success rate was 73% for azithromycin and 71% for the control agent.
Trial 2
In a non-comparative clinical and microbiologic trial performed in the United States, where significant rates of beta-lactamase producing organisms (35%) were found, 131 patients were evaluable for clinical efficacy. The combined clinical success rate (i.e., cure and improvement) at the Day 11 visit was 84% for azithromycin. For the 122 patients who were evaluated at the Day 30 visit, the clinical success rate was 70% for azithromycin.
Microbiologic determinations were made at the pre-treatment visit. Microbiology was not reassessed at later visits. The following clinical success rates were obtained from the evaluable group:
Day 11
Day 30
Pathogen
Azithromycin
Azithromycin
S. pneumoniae
61/74 (82%)
40/56 (71%)
H. influenzae
43/54 (80%)
30/47 (64%)
M. catarrhalis
28/35 (80%)
19/26 (73%)
S. pyogenes
11/11 (100%)
7/7 (100%)
Overall
177/217 (82%)
97/137 (73%)
In another controlled comparative clinical and microbiologic study of otitis media performed in the United States, azithromycin (10 mg/kg on Day 1 followed by 5 mg/kg on Days 2 to 5) was compared to amoxicillin/clavulanate potassium (4:1). This study utilized two of the same investigators as Protocol 2 (above), and these two investigators enrolled 90% of the patients in Protocol 3. For this reason, Protocol 3 was not considered to be an independent study. Significant rates of beta-lactamase producing organisms (20%) were found. Ninety-two (92) patients were evaluable for clinical and microbiologic efficacy. The combined clinical success rate (i.e., cure and improvement) of those patients with a baseline pathogen at the Day 11 visit was 88% for azithromycin vs. 100% for control; at the Day 30 visit, the clinical success rate was 82% for azithromycin vs. 80% for control.
Microbiologic determinations were made at the pre-treatment visit. Microbiology was not reassessed at later visits. At the Day 11 and Day 30 visits, the following clinical success rates were obtained from the evaluable group:
Day 11
Day 30
Pathogen
Azithromycin
Control
Azithromycin
Control
S. pneumoniae
25/29 (86%)
26/26 (100%)
22/28 (79%)
18/22 (82%)
H. influenzae
9/11 (82%)
9/9 (100%)
8/10 (80%)
6/8 (75%)
M. catarrhalis
7/7 (100%)
5/5 (100%)
5/5 (100%)
2/3 (66%)
S. pyogenes
2/2 (100%)
5/5 (100%)
2/2 (100%)
4/4 (100%)
Overall
43/49 (88%)
45/45 (100%)
37/45 (82%)
30/37 (81%)
Efficacy using azithromycin given over 3 days (10 mg/kg/day):
Trial 4
In a double-blind, controlled, randomized clinical study of acute otitis media in pediatric patients from 6 months to 12 years of age, azithromycin (10 mg/kg per day for 3 days) was compared to amoxicillin/clavulanate potassium (7:1) in divided doses q12h for 10 days. Each patient received active drug and placebo matched for the comparator.
For the 366 patients who were evaluated for clinical efficacy at the Day 12 visit, the clinical success rate (i.e., cure plus improvement) was 83% for azithromycin and 88% for the control agent. For the 362 patients who were evaluated at the Days 24 to 28 visit, the clinical success rate was 74% for azithromycin and 69% for the control agent.
Efficacy using azithromycin 30 mg/kg given as a single dose:
Trial 5
A double-blind, controlled, randomized trial was performed at nine clinical centers. Pediatric patients from 6 months to 12 years of age were randomized 1:1 to treatment with either azithromycin (given at 30 mg/kg as a single dose on Day 1) or amoxicillin/clavulanate potassium (7:1), divided q12h for 10 days. Each child received active drug, and placebo matched for the comparator.
Clinical response (Cure, Improvement, Failure) was evaluated at End of Therapy (Days 12 to 16) and Test of Cure (Days 28 to 32). Safety was evaluated throughout the trial for all treated subjects. For the 321 subjects who were evaluated at End of Treatment, the clinical success rate (cure plus improvement) was 87% for azithromycin, and 88% for the comparator. For the 305 subjects who were evaluated at Test of Cure, the clinical success rate was 75% for both azithromycin and the comparator.
Trial 6
In a non-comparative clinical and microbiological trial, 248 patients from 6 months to 12 years of age with documented acute otitis media were dosed with a single oral dose of azithromycin (30 mg/kg on Day 1).
For the 240 patients who were evaluable for clinical modified Intent-to-Treat (MITT) analysis, the clinical success rate (i.e., cure plus improvement) at Day 10 was 89% and for the 242 patients evaluable at Days 24 to 28, the clinical success rate (cure) was 85%.
Presumed Bacteriologic Eradication
Day 10
Days 24-28
S. pneumoniae
70/76 (92%)
67/76 (88%)
H. influenzae
30/42 (71%)
28/44 (64%)
M. catarrhalis
10/10 (100%)
10/10 (100%)
Overall
110/128 (86%)
105/130 (81%)
-
16 HOW SUPPLIED/STORAGE AND HANDLINGAzithromycin tablets USP are supplied in the following strengths and package configurations: Azithromycin tablets USP, 250 mg are supplied as pink, oval shaped film-coated tablets, engraved with ...
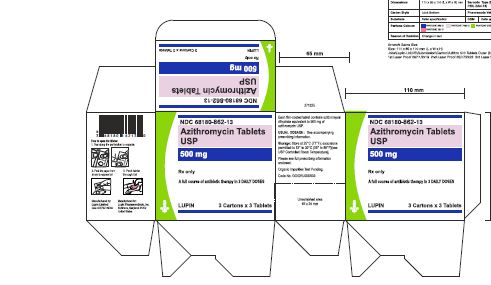
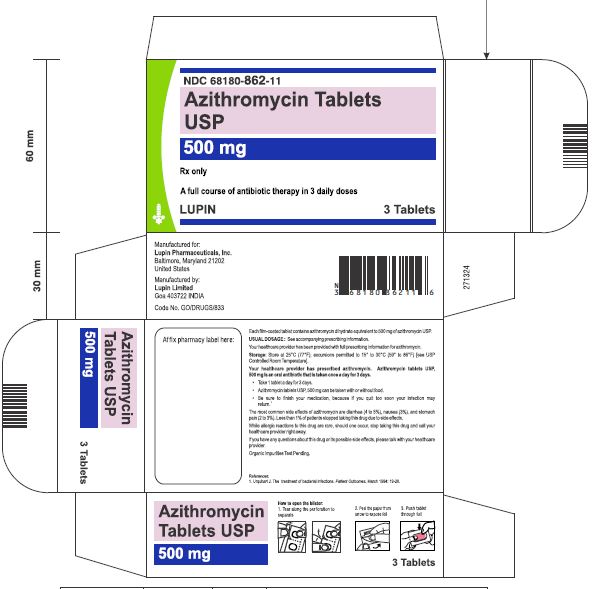
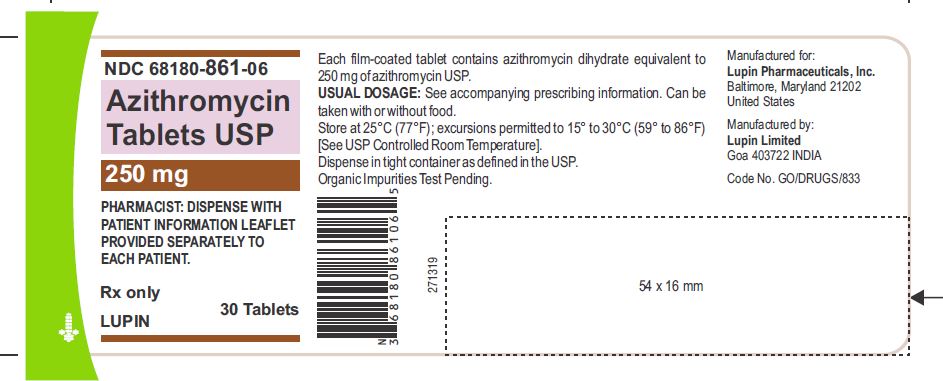
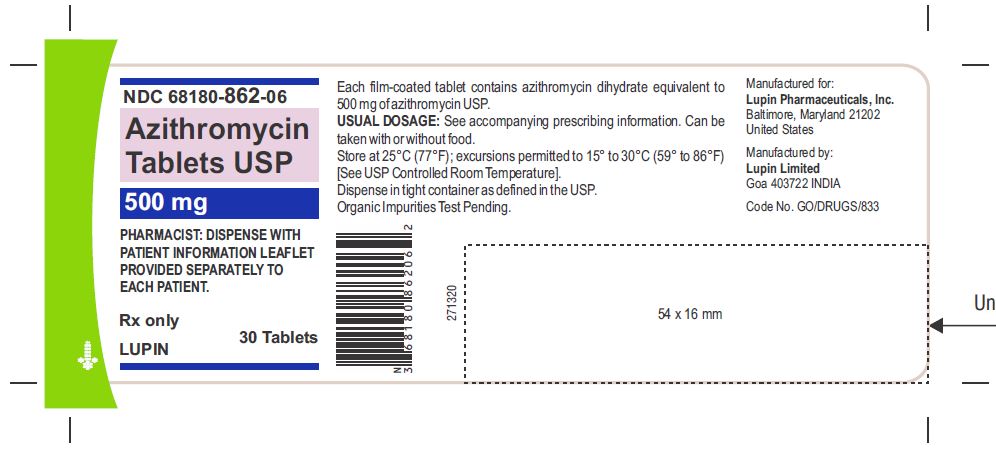
Azithromycin tablets USP are supplied in the following strengths and package configurations:
Azithromycin tablets USP, 250 mg are supplied as pink, oval shaped film-coated tablets, engraved with "LU" on one side and "L04" on the other side containing azithromycin dihydrate equivalent to 250 mg of azithromycin USP.
These are packaged in bottles and blister cards as follows:
Bottles of 30 Tablets NDC 68180-861-06
Carton of 1 Blister Card (6 Tablets per Blister Card) [1 x 6 Tablets] NDC 68180-861-11
Carton of 3 Blister Cards (6 Tablets per Blister Card) [3 x 6 Tablets] NDC 68180-861-13
Azithromycin tablets USP, 500 mg are supplied as pink, oval shaped film-coated tablets, engraved with "LU" on one side and "L05" on the other side containing azithromycin dihydrate equivalent to 500 mg of azithromycin USP.
These are packaged in bottles and blister cards as follows:
Bottles of 30 Tablets NDC 68180-862-06
Carton of 1 Blister Card (3 Tablets per Blister Card) [1 x 3 Tablets] NDC 68180-862-11
Carton of 3 Blister Cards (3 Tablets per Blister Card) [3 x 3 Tablets] NDC 68180-862-13
Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) [See USP Controlled Room Temperature].
Close -
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). General Patient Counseling - Azithromycin tablets can be taken with or without food. Patients should also be ...
Advise the patient to read the FDA-approved patient labeling (Patient Information).
General Patient Counseling
Azithromycin tablets can be taken with or without food.
Patients should also be cautioned not to take aluminum-and magnesium-containing antacids and azithromycin simultaneously.
The patient should be directed to discontinue azithromycin immediately and contact a physician if any signs of an allergic reaction occur.
Direct parents or caregivers to contact their physician if vomiting and irritability with feeding occurs in the infant.
Patients should be counseled that antibacterial drugs including azithromycin should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When azithromycin is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of the therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by azithromycin or other antibacterial drugs in the future.
Diarrhea is a common problem caused by antibacterials which usually ends when the antibacterial is discontinued. Sometimes after starting treatment with antibacterials patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibacterial drug. If this occurs, patients should contact their physician as soon as possible.
Manufactured for:
Lupin Pharmaceuticals, Inc.
Baltimore, Maryland 21202
United States
Manufactured by:
Lupin Limited
Goa - 403722
India
Revised: August 2022
Close -
PATIENT PACKAGE INSERTPatient Information - Azithromycin (ay-ZITH-roe-MYE-sin) Tablets USP - Read this Patient Information leaflet before you start taking azithromycin tablets and each time you get a refill. There may ...
Azithromycin (ay-ZITH-roe-MYE-sin) Tablets USP
Read this Patient Information leaflet before you start taking azithromycin tablets and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
What are azithromycin tablets?
Azithromycin tablets are a macrolide antibiotic prescription medicine used in adults 18 years or older to treat certain infections caused by certain germs called bacteria. These bacterial infections include:
- acute worsening of chronic bronchitis
- acute sinus infection
- community-acquired pneumonia
- infected throat or tonsils
- skin infections
- infections of the urethra or cervix
- genital ulcers in men
Azithromycin tablets are also used in children to treat:
- ear infections
- community-acquired pneumonia
- infected throat or tonsils
Azithromycin should not be taken by people who cannot tolerate oral medications because they are very ill or have certain other risk factors including:
- have cystic fibrosis
- have hospital acquired infections
- have known or suspected bacteria in the blood
- need to be in the hospital
- are elderly
- have any medical problems that can lower the ability of the immune system to fight infections
Azithromycin tablets are not for viral infections such as the common cold.
It is not known if azithromycin tablets are safe and effective for genital ulcers in women.
It is not known if azithromycin tablets are safe and effective for children with ear infections, sinus infections, and community-acquired pneumonia under 6 months of age.
It is not known if azithromycin tablets are safe and effective for infected throat or tonsils in children under 2 years of age.
Who should not take azithromycin tablets?
Do not take azithromycin tablets if you:
- have had a severe allergic reaction to certain antibiotics known as macrolides or ketolides including azithromycin and erythromycin.
- have a history of cholestatic jaundice or hepatic dysfunction that happened with the use of azithromycin.
What should I tell my healthcare provider before taking azithromycin tablets?
Before you take azithromycin tablets, tell your healthcare provider if you:
- have pneumonia
- have cystic fibrosis
- have known or suspected bacteremia (bacterial infection in the blood)
- have liver or kidney problems
- have an irregular heartbeat, especially a problem called "QT prolongation"
- have a problem that causes muscle weakness (myasthenia gravis)
- have any other medical problems
- are pregnant or plan to become pregnant. It is not known if azithromycin tablets will harm your unborn baby.
- are breastfeeding or plan to breastfeed. Azithromycin has been reported to pass into breast milk. Talk to your healthcare provider about the best way to feed your baby while you take azithromycin tablets.
Contact your healthcare provider immediately if you are giving azithromycin tablets to a young child (less than 6 weeks of age) and he or she vomits or becomes irritable when fed.
Tell your healthcare provider about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements.
Azithromycin tablets and other medicines may affect each other causing side effects. Azithromycin tablets may affect the way other medicines work, and other medicines may affect how azithromycin tablets works.
Especially tell your healthcare provider if you take:
- nelfinavir
- a blood thinner (warfarin)
- digoxin
- colchicine
- phenytoin
- an antacid that contains aluminum or magnesium
Know the medicines you take. Keep a list of your medicines and show it to your healthcare provider and pharmacist when you get a new medicine.
How should I take azithromycin tablets?
- Take azithromycin tablets exactly as your healthcare provider tells you to take it.
- Azithromycin tablets can be taken with or without food.
- Do not skip any doses of azithromycin tablets or stop taking it, even if you begin to feel better, until you finish your prescribed treatment unless you have a serious allergic reaction or your healthcare provider tells you to stop taking azithromycin tablets. "See What are the possible side effects of azithromycin tablets?" If you skip doses, or do not complete the total course of azithromycin tablets your treatment may not work as well and your infection may be harder to treat. Taking all of your azithromycin tablets doses will help lower the chance that the bacteria will become resistant to azithromycin tablets.
- If the bacteria becomes resistant to azithromycin, azithromycin tablets and other antibiotic medicines may not work for you in the future.
- If you take too much azithromycin tablets, call your healthcare provider or get medical help right away.
What are the possible side effects of azithromycin tablets?
Azithromycin tablets can cause serious side effects, including:
• Serious allergic reactions. Allergic reactions can happen in people taking azithromycin tablets the active ingredient in azithromycin tablets, even after only 1 dose. Stop taking azithromycin tablets and get emergency medical help right away if you have any of the following symptoms of a severe allergic reaction:
° trouble breathing or swallowing
° swelling of the lips, tongue, face
° throat tightness, hoarseness
° rapid heartbeat
° faintness
° skin rash (hives)
° new onset of fever and swollen lymph nodes
Stop taking azithromycin tablets at the first sign of a skin rash and call your healthcare provider.
Skin rash may be a sign of a more serious reaction to azithromycin tablets.
• Liver damage (hepatotoxicity). Hepatotoxicity can happen in people who take azithromycin tablets. Call your healthcare provider right away if you have unexplained symptoms such as:
° nausea or vomiting
° stomach pain
° fever
° weakness
° abdominal pain or tenderness
° itching
° unusual tiredness
° loss of appetite
° change in the color of your bowel movements
° dark colored urine
° yellowing of your skin or of the whites of your eyes
Stop taking azithromycin tablets and tell your healthcare provider right away if you have yellowing of your skin or white part of your eyes, or if you have dark urine. These can be signs of a serious reaction to azithromycin tablets (a liver problem).
• Serious heart rhythm changes that can be life-threatening, including heart stopping (cardiac arrest), QT prolongation, torsades de pointes, feeling that your heart is pounding or racing (palpitations), chest discomfort, or irregular heartbeat.
Tell your healthcare provider right away if you or your child feel a fast or irregular heartbeat, get dizzy or faint. Azithromycin tablets may cause a rare heart problem known as prolongation of the QT interval. This condition can cause an abnormal heartbeat and can be very dangerous. The chances of this happening are higher in people:
° who are elderly
° with a family history of prolonged QT interval
° with low blood potassium
° who take certain medicines to control heart rhythm (antiarrhythmics)
• Worsening of myasthenia gravis (a problem that causes muscle weakness).
Certain antibiotics like azithromycin tablets may cause worsening of myasthenia gravis symptoms, including muscle weakness and breathing problems. Call your healthcare provider right away if you have any worsening muscle weakness or breathing problems.
• Diarrhea. Tell your healthcare provider right away if you have watery diarrhea, diarrhea that does not go away, or bloody stools. You may experience cramping and a fever. This could happen after you have finished your azithromycin tablets.
The most common side effects of azithromycin tablets include:
•nausea
•stomach pain
•vomiting
These are not all the possible side effects of azithromycin tablets. Tell your healthcare provider about any side effect that bothers you or that does not go away.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store azithromycin tablets?
- Store azithromycin tablets at 15° to 30°C (59° to 86°F).
- Safely throw away any medicine that is out of date or no longer needed.
Keep azithromycin tablets and all medicines out of the reach of children.
General information about the safe and effective use of azithromycin tablets.
Medicines are sometimes prescribed for purposes other than those listed in the Patient Information leaflet. Do not use azithromycin tablets for a condition for which it was not prescribed. Do not give azithromycin tablets to other people, even if they have the same symptoms you have. It may harm them.
This Patient Information leaflet summarizes the most important information about azithromycin tablets. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about azithromycin tablets that is written for health professionals.
For more information, go to www.lupinpharmaceuticals.com or call 1-800-399-2561
What are the ingredients in azithromycin tablets?
Azithromycin Tablets:
Active ingredient: azithromycin dihydrate
Inactive ingredients: croscarmellose sodium, dibasic calcium phosphate, hydroxypropyl methyl cellulose, lactose monohydrate, magnesium stearate, sodium lauryl sulfate, titanium dioxide, triacetin and D & C Red #30.
How to open the blister:
This Patient Information has been approved by the U.S. Food and Drug Administration.
Manufactured for:
Lupin Pharmaceuticals, Inc.
Baltimore, Maryland 21202
United States
Manufactured by:
Lupin Limited
Goa - 403722
India
Revised: August 2022 ID#: 275212
Close -
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Azithromycin Tablets USP, 250 mg - Container Label-30 Tablets - NDC 68180-861-06 - Azithromycin Tablets USP, 250 mg - 3 Card x 6 Tablets- Outer Carton Label - NDC 68180-861-13 - Azithromycin ...
Azithromycin Tablets USP, 250 mg
3 Card x 6 Tablets- Outer Carton Label
NDC 68180-861-13
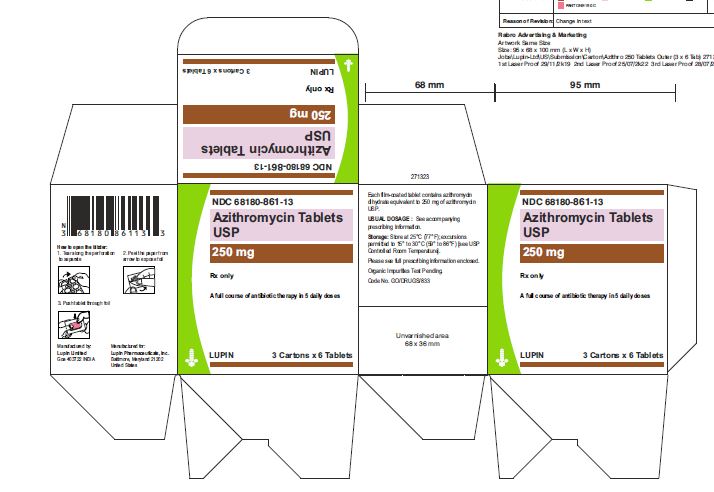
Azithromycin Tablets USP, 250 mg
1 Card x 6 Tablets- Mono Carton Label
NDC 68180-861-11
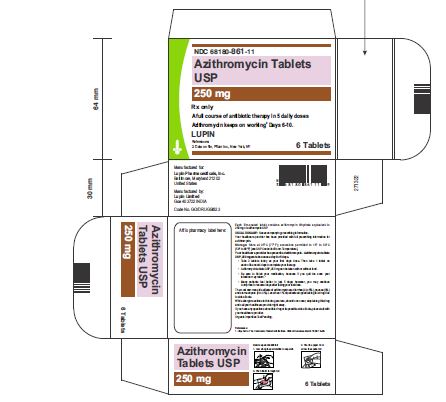 Close
CloseAzithromycin Tablets USP, 500 mg
3 Card x 3 Tablets- Outer Carton Label
NDC 68180-862-13
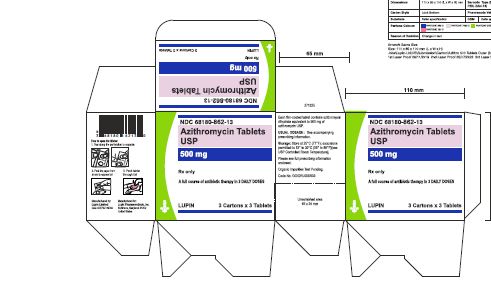
Azithromycin Tablets USP, 500 mg
1 Card x 3 Tablets- Mono Carton Label
NDC 68180-862-11
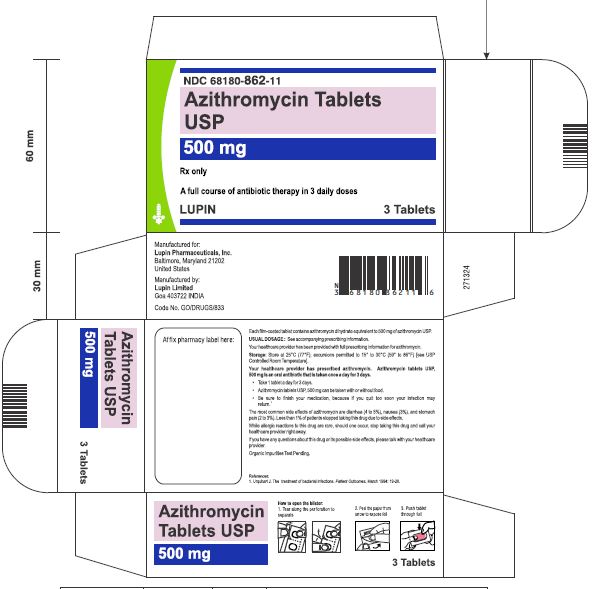
-
INGREDIENTS AND APPEARANCEProduct Information
AZITHROMYCIN DIHYDRATE azithromycin dihydrate tablet, film coated Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:68180-861 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AZITHROMYCIN DIHYDRATE (UNII: 5FD1131I7S) (AZITHROMYCIN ANHYDROUS - UNII:J2KLZ20U1M) AZITHROMYCIN ANHYDROUS 250 mg Inactive Ingredients Ingredient Name Strength CALCIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: L11K75P92J) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) D&C RED NO. 30 (UNII: 2S42T2808B) HYPROMELLOSE 2910 (15 MPA.S) (UNII: 36SFW2JZ0W) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) SODIUM LAURYL SULFATE (UNII: 368GB5141J) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) Product Characteristics Color PINK Score no score Shape OVAL Size 14mm Flavor Imprint Code LU;L04 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68180-861-11 6 in 1 BLISTER PACK; Type 0: Not a Combination Product 11/17/2022 2 NDC:68180-861-06 30 in 1 CONTAINER; Type 0: Not a Combination Product 11/17/2022 3 NDC:68180-861-13 3 in 1 CARTON 01/01/2040 3 6 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA065398 11/17/2022 AZITHROMYCIN DIHYDRATE azithromycin dihydrate tablet, film coated Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:68180-862 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AZITHROMYCIN DIHYDRATE (UNII: 5FD1131I7S) (AZITHROMYCIN ANHYDROUS - UNII:J2KLZ20U1M) AZITHROMYCIN ANHYDROUS 500 mg Inactive Ingredients Ingredient Name Strength CALCIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: L11K75P92J) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) D&C RED NO. 30 (UNII: 2S42T2808B) HYPROMELLOSE 2910 (15 MPA.S) (UNII: 36SFW2JZ0W) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) SODIUM LAURYL SULFATE (UNII: 368GB5141J) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) Product Characteristics Color PINK Score no score Shape OVAL Size 17mm Flavor Imprint Code LU;L05 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68180-862-11 1 in 1 CARTON 11/17/2022 1 3 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:68180-862-06 30 in 1 CONTAINER; Type 0: Not a Combination Product 11/17/2022 3 NDC:68180-862-13 3 in 1 CARTON 01/01/2040 3 3 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA065399 08/18/2015 Labeler - Lupin Pharmaceuticals, Inc. (089153071) Registrant - LUPIN LIMITED (675923163)
CloseEstablishment Name Address ID/FEI Business Operations LUPIN LIMITED 677600414 MANUFACTURE(68180-861, 68180-862) , PACK(68180-861, 68180-862)
Find additional resources
(also available in the left menu)Safety
Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
View Labeling Archives for this drug
AZITHROMYCIN DIHYDRATE tablet, film coated
Number of versions: 4
| Published Date (What is this?) | Version | Files |
|---|---|---|
| Feb 28, 2025 | 4 (current) | download |
| Dec 8, 2023 | 3 | download |
| Oct 5, 2023 | 2 | download |
| Nov 29, 2022 | 1 | download |
RxNorm
AZITHROMYCIN DIHYDRATE tablet, film coated
| RxCUI | RxNorm NAME | RxTTY | |
|---|---|---|---|
| 1 | 248656 | azithromycin 500 MG Oral Tablet | PSN |
| 2 | 248656 | azithromycin 500 MG Oral Tablet | SCD |
| 3 | 248656 | azithromycin 500 MG (as azithromycin monohydrate) Oral Tablet | SY |
| 4 | 308460 | azithromycin 250 MG Oral Tablet | PSN |
| 5 | 308460 | azithromycin 250 MG Oral Tablet | SCD |
| 6 | 749780 | azithromycin 500 MG Oral Tablet 3 Count Pack | PSN |
| 7 | 749780 | {3 (azithromycin 500 MG Oral Tablet) } Pack | GPCK |
| 8 | 749780 | azithromycin 500 MG Oral Tablet 3 Count Pack | SY |
| 9 | 749783 | azithromycin 250 MG Oral Tablet 6 Count Pack | PSN |
| 10 | 749783 | {6 (azithromycin 250 MG Oral Tablet) } Pack | GPCK |
| 11 | 749783 | azithromycin 250 MG Oral Tablet 6 Count Pack | SY |
Get Label RSS Feed for this Drug
AZITHROMYCIN DIHYDRATE tablet, film coated
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=01649d79-4677-4577-a3e4-e054f3f9fc3b
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
AZITHROMYCIN DIHYDRATE tablet, film coated
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 68180-861-06 |
| 2 | 68180-861-11 |
| 3 | 68180-861-13 |
| 4 | 68180-862-06 |
| 5 | 68180-862-11 |
| 6 | 68180-862-13 |