Label: DESLORATADINE tablet, film coated
- NDC Code(s): 68180-153-01, 68180-153-02, 68180-153-03, 68180-153-06, view more
- Packager: Lupin Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 19, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use DESLORATADINE TABLETS safely and effectively. See full prescribing information for DESLORATADINE TABLETS. DESLORATADINE tablets for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Seasonal Allergic Rhinitis - Desloratadine tablets are indicated for the relief of the nasal and non-nasal symptoms of seasonal allergic rhinitis in patients 12 years of age and ...
-
2 DOSAGE AND ADMINISTRATIONDesloratadine tablets may be taken without regard to meals. 2.1 Adults and Adolescents 12 Years of Age and Over - The recommended dose of desloratadine tablets is one 5 mg tablet once ...
-
3 DOSAGE FORMS AND STRENGTHSDesloratadine tablets USP, 5 mg are light blue, circular, biconvex, film-coated tablets, debossed "LU" on one side and "S71" on other side.
-
4 CONTRAINDICATIONSDesloratadine tablets are contraindicated in patients who are hypersensitive to this medication or to any of its ingredients or to loratadine [see WARNINGS AND PRECAUTIONS (5.1) and ADVERSE ...
-
5 WARNINGS AND PRECAUTIONS5.1 Hypersensitivity Reactions - Hypersensitivity reactions including rash, pruritus, urticaria, edema, dyspnea, and anaphylaxis have been reported after administration of desloratadine. If such ...
-
6 ADVERSE REACTIONSThe following adverse reactions are discussed in greater detail in other sections of the label: Hypersensitivity reactions. [See WARNINGS AND PRECAUTIONS (5.1).] 6.1 Clinical Trials ...
-
7 DRUG INTERACTIONS7.1 Inhibitors of Cytochrome P450 3A4 - In controlled clinical studies co-administration of desloratadine with ketoconazole, erythromycin, or azithromycin resulted in increased plasma ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - The limited available data with desloratadine in pregnant women are not sufficient to inform a drug-associated risk for major birth defects and miscarriage. There ...
-
9 DRUG ABUSE AND DEPENDENCEThere is no information to indicate that abuse or dependency occurs with desloratadine tablets.
-
10 OVERDOSAGEIn the event of overdose, consider standard measures to remove any unabsorbed drug. Symptomatic and supportive treatment is recommended. Desloratadine and 3-hydroxydesloratadine are not ...
-
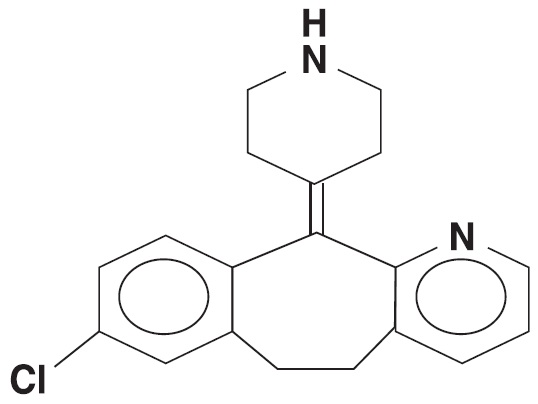
11 DESCRIPTIONDesloratadine tablets USP, 5 mg are light blue, circular, biconvex, film-coated tablets debossed "LU" on one side and "S71" on other side, containing 5 mg desloratadine, an antihistamine, to ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Desloratadine is a long-acting tricyclic histamine antagonist with selective H1-receptor histamine antagonist activity. Receptor binding data indicates that at a ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenicity Studies - The carcinogenic potential of desloratadine was assessed using a loratadine study in rats and a ...
-
14 CLINICAL STUDIES14.1 Seasonal Allergic Rhinitis - The clinical efficacy and safety of desloratadine tablets were evaluated in over 2300 patients 12 to 75 years of age with seasonal allergic rhinitis. A total of ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGDesloratadine tablets USP, 5 mg are light blue, circular, biconvex, film-coated tablets, debossed "LU" on one side and "S71" on other side. They are supplied as follows: NDC ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). 17.1 Information for Patients - Patients should be instructed to use desloratadine tablets as directed ...
-
PATIENT PACKAGE INSERTPATIENT INFORMATION LEAFLET - DESLORATADINE (DES-lor-A-ta-deen) TABLETS USP - Rx only - Read the Patient Information that comes with desloratadine tablets before you start taking it and each ...
-
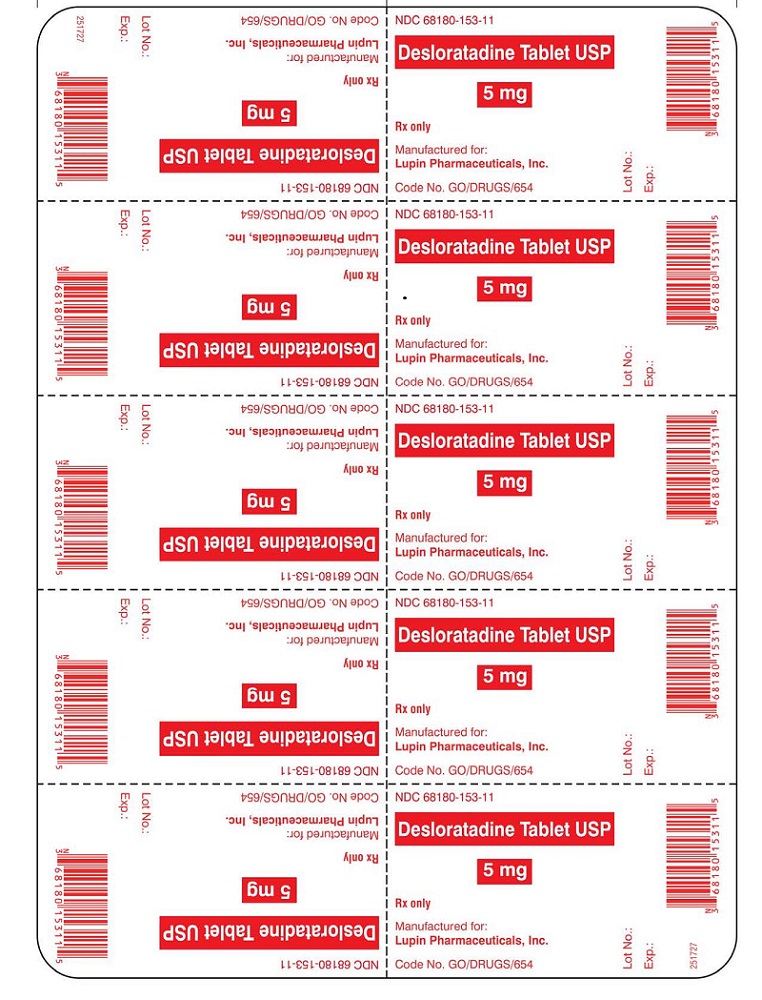
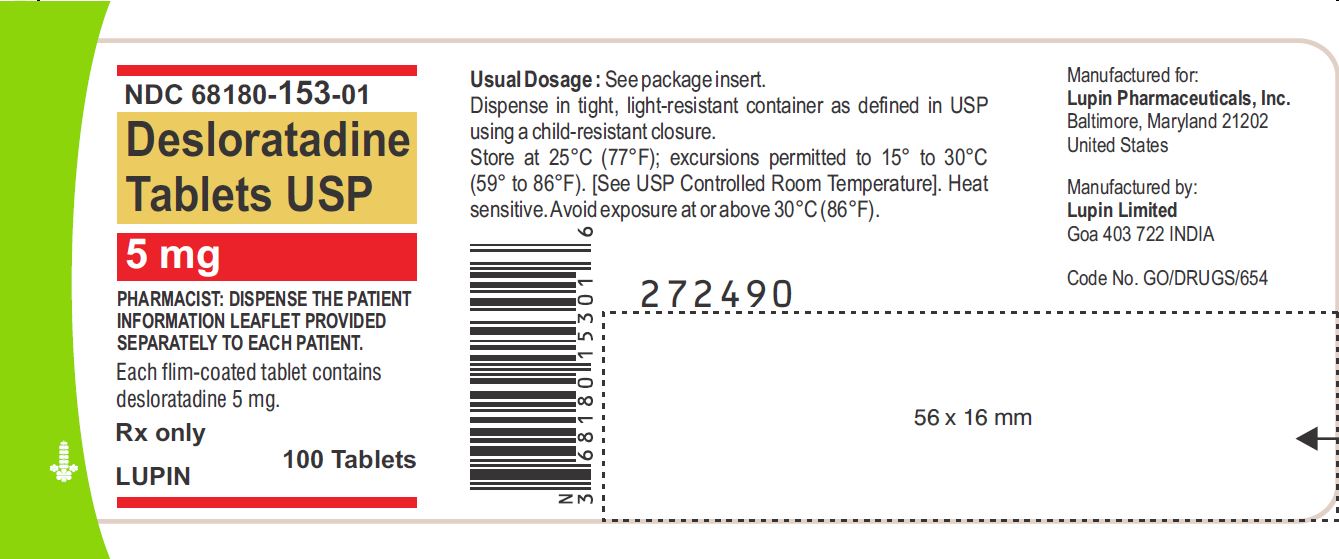
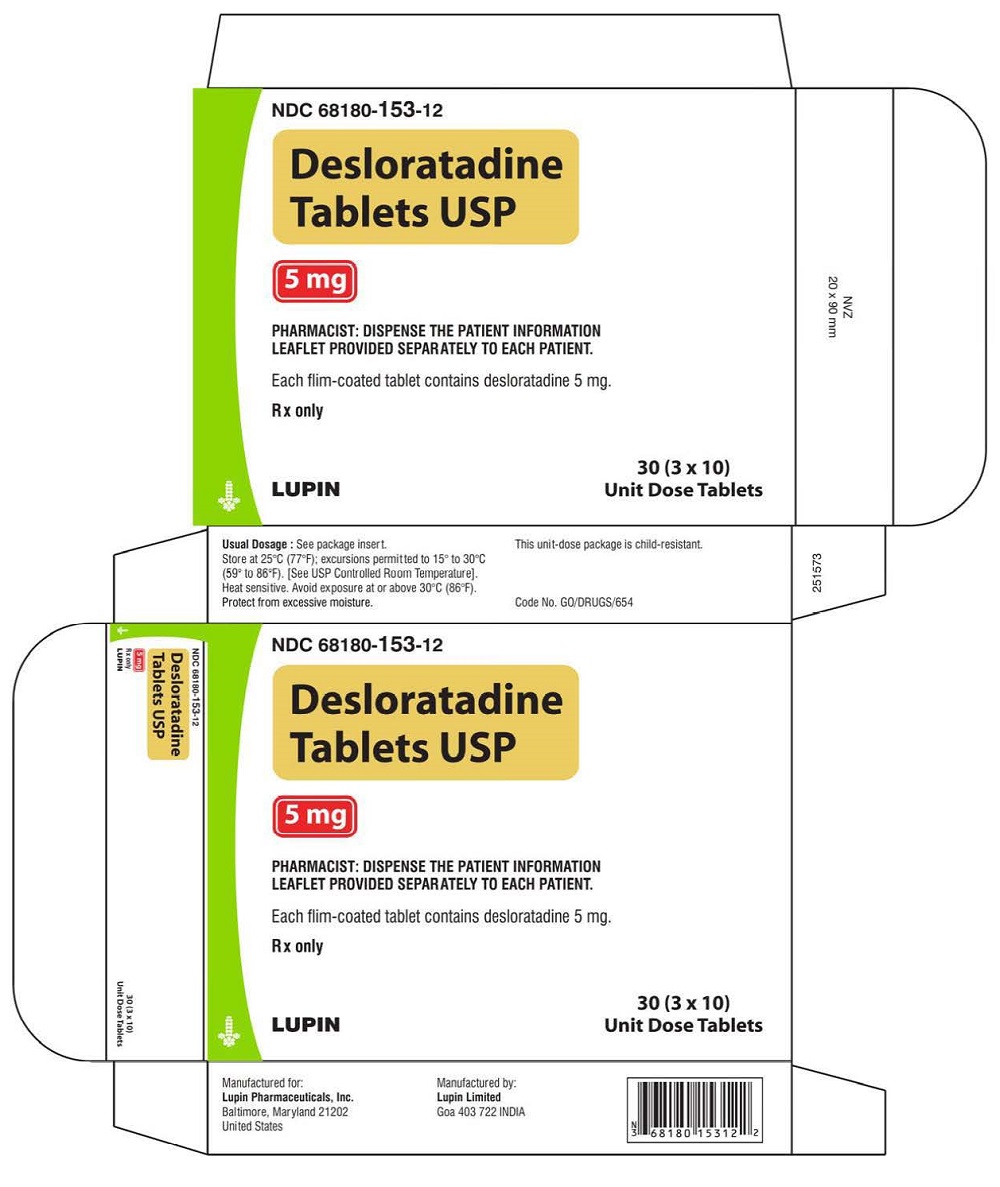
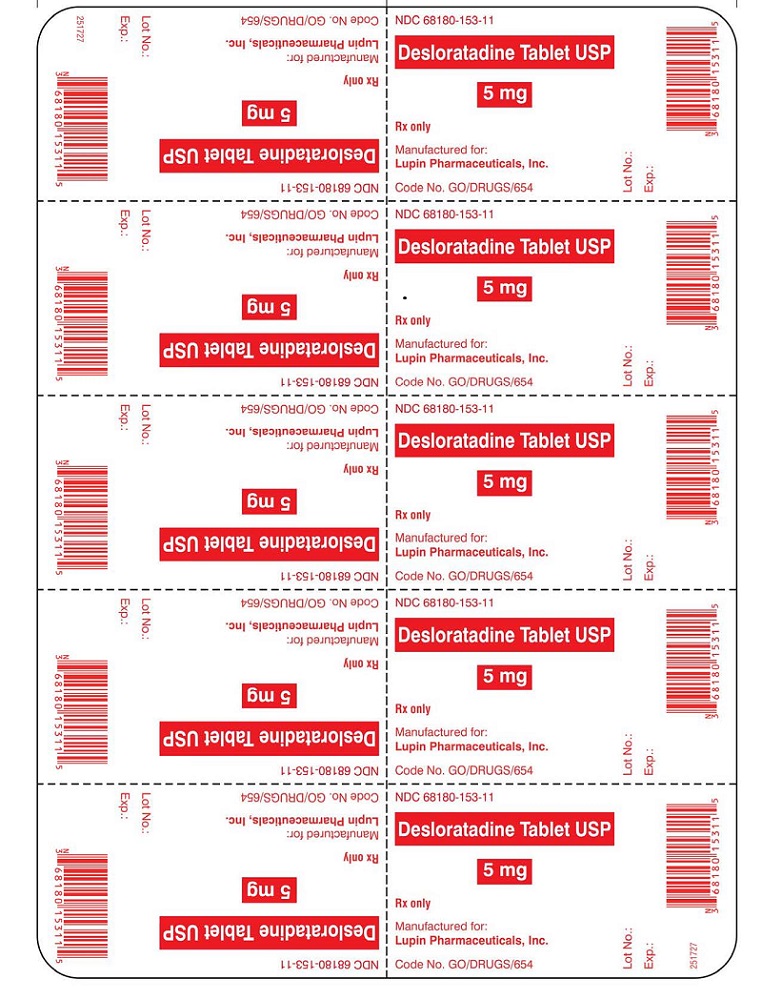
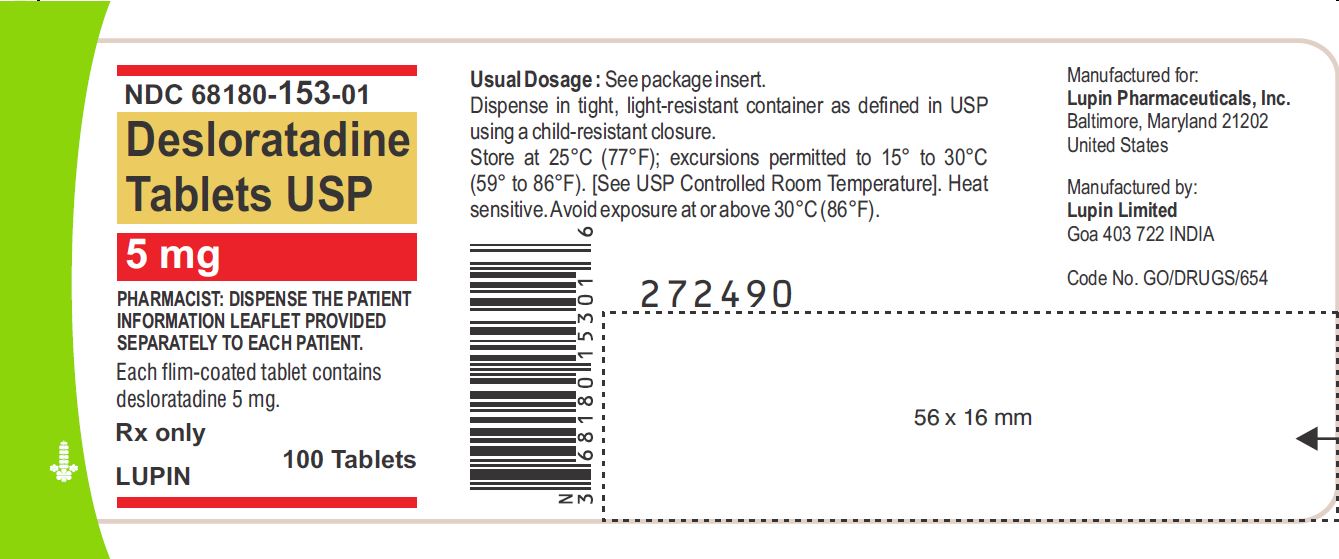
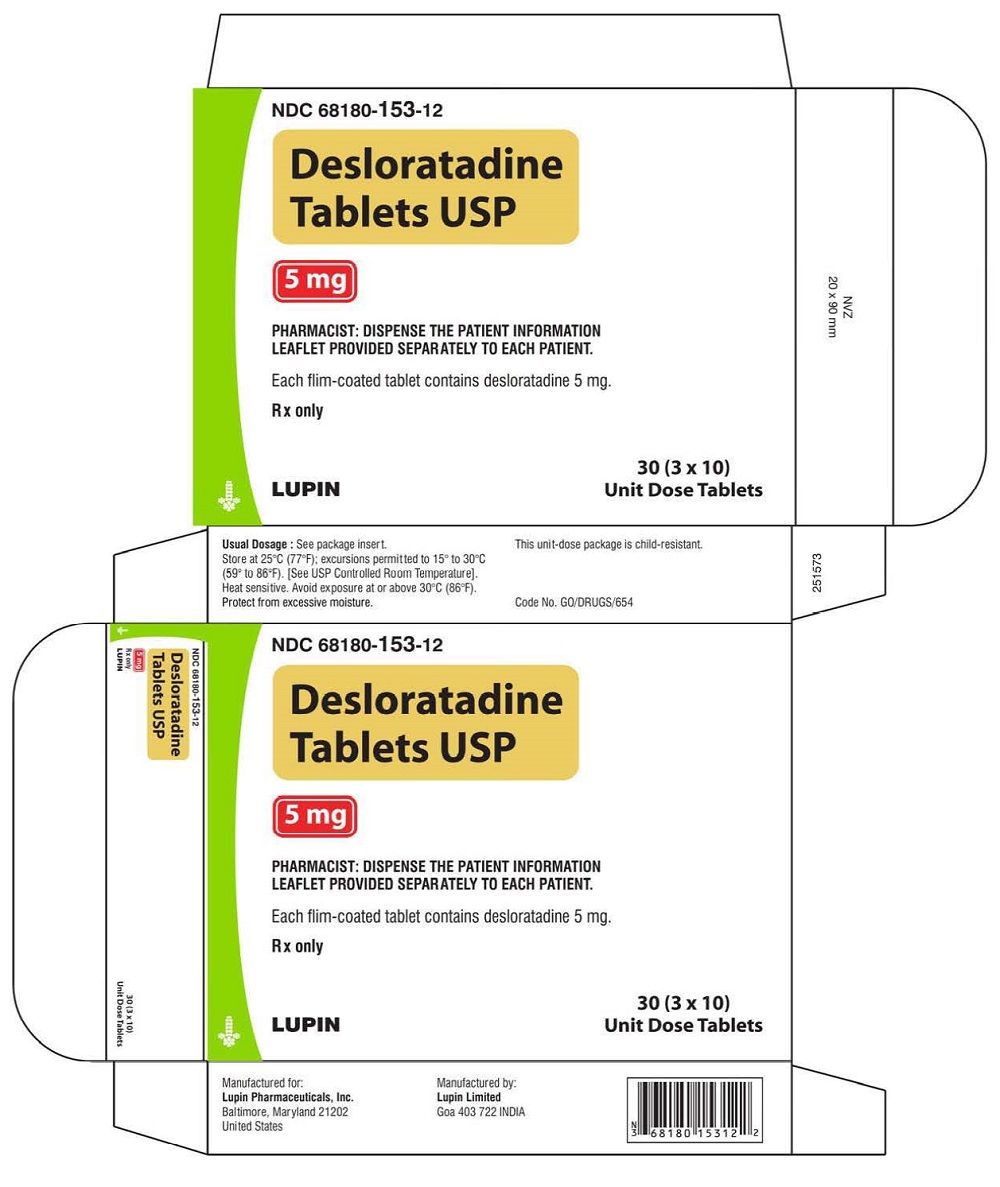
PRINCIPAL DISPLAY PANELDesloratadine Tablets - Rx Only - 5 mg - NDC 68180-153-11 - BLISTER FOIL LABEL - 10 TABLETS SINGLE UNIT PACKAGE - Desloratadine Tablets - Rx Only - 5 mg - NDC 68180-153-01 - BOTTLE LABEL - 100 ...
-
INGREDIENTS AND APPEARANCEProduct Information