Label: TIZANIDINE tablet
- NDC Code(s): 67877-613-05, 67877-613-15, 67877-614-05, 67877-614-15
- Packager: Ascend Laboratories, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 13, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use TIZANIDINE TABLETS safely and effectively. See full prescribing information for TIZANIDINE TABLETS. TIZANIDINE tablets, for oral ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGETizanidine is a central alpha-2-adrenergic agonist indicated for the management of spasticity. Because of the short duration of therapeutic effect, treatment with tizanidine should be reserved for ...
-
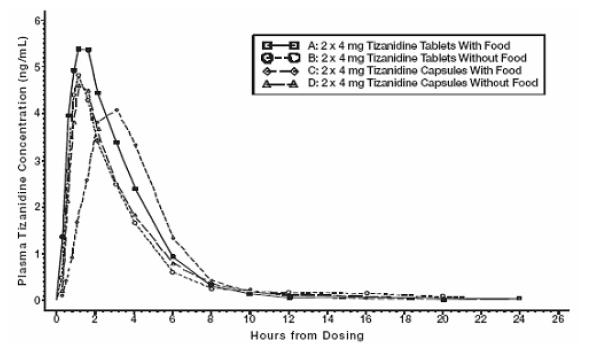
2 DOSAGE AND ADMINISTRATION2.1 Dosing Information - Tizanidine tablets may be prescribed with or without food. Once the formulation has been selected and the decision to take with or without food has been made, this ...
-
3 DOSAGE FORMS AND STRENGTHSTablets - 2 mg White to off-white round uncoated tablets with score on one side and debossed with “2” below “TN” on other side - 4 mg White to off-white round uncoated tablets with quadrisecting ...
-
4 CONTRAINDICATIONSTizanidine is contraindicated in patients taking potent inhibitors of CYP1A2, such as fluvoxamine or ciprofloxacin [see Drug Interactions (7.1, 7.2)].
-
5 WARNINGS AND PRECAUTIONS5.1 Hypotension - Tizanidine is an α2-adrenergic agonist that can produce hypotension. Syncope has been reported in the post marketing setting. The chance of significant hypotension may possibly ...
-
6 ADVERSE REACTIONSThe following adverse reactions are described elsewhere in other sections of the prescribing information: Hypotension [see Warnings and Precautions (5.1)]. Liver Injury [see Warnings and ...
-
7 DRUG INTERACTIONS7.1 Fluvoxamine - Concomitant use of fluvoxamine and tizanidine is contraindicated. Changes in pharmacokinetics of tizanidine when administered with fluvoxamine resulted in significantly ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Pregnancy Category C - Tizanidine has not been studied in pregnant women. Tizanidine should be given to pregnant women only if the benefit outweighs the risk to the unborn fetus ...
-
9 DRUG ABUSE AND DEPENDENCE9.2 Abuse - Abuse potential was not evaluated in human studies. Rats were able to distinguish tizanidine from saline in a standard discrimination paradigm, after training, but failed to ...
-
10 OVERDOSAGEA review of the safety surveillance database revealed cases of intentional and accidental tizanidine overdose. Some of the cases resulted in fatality and many of the intentional overdoses were ...
-
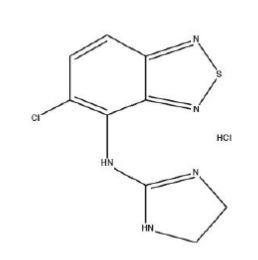
11 DESCRIPTIONTizanidine hydrochloride is a central alpha2-adrenergic agonist. Tizanidine hydrochloride is almost white to slightly yellow crystalline powder. Tizanidine is slightly soluble in water and ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Tizanidine is a central alpha-2-adrenergic receptor agonist and presumably reduces spasticity by increasing presynaptic inhibition of motor neurons. The effects of ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, mutagenesis, impairment of fertility - Carcinogenesis - Tizanidine was administered to mice for 78 weeks at oral doses up to 16 mg/kg/day, which is 2 times the maximum ...
-
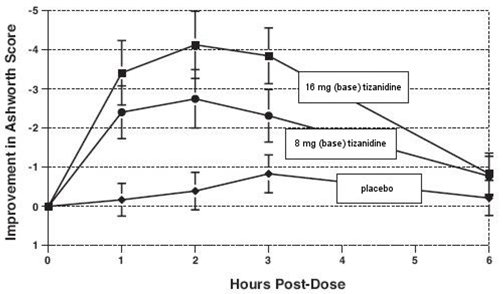
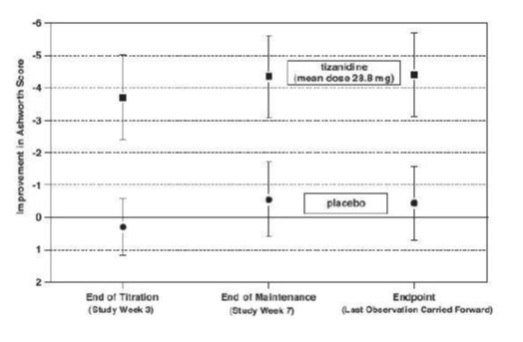
14 CLINICAL STUDIESTizanidine’s capacity to reduce increased muscle tone associated with spasticity was demonstrated in two adequate and well controlled studies in patients with multiple sclerosis or spinal cord ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGTizanidine Tablets, USP 2 mg are available as White to off-white round uncoated tablets with score on one side and debossed with "2" below "TN" on other side. Tablets are supplied as ...
-
17 PATIENT COUNSELING INFORMATIONSerious Drug Interactions - Advise patients they should not take tizanidine if they are taking fluvoxamine or ciprofloxacin because of the increased risk of serious adverse reactions including ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELPrincipal display panel- 2 mg bottle label - NDC 67877-613-15 - Tizanidine Tablets, USP - 2 mg - Rx Only - 150 Tablets - STOP: Tizanidine Tablets are not interchangeable with Tizanidine Cpasules ...
-
INGREDIENTS AND APPEARANCEProduct Information