Label: CEFPODOXIME PROXETIL tablet, film coated
- NDC Code(s): 67877-559-01, 67877-559-05, 67877-559-20, 67877-560-01, view more
- Packager: Ascend Laboratories, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 20, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONCefpodoxime Proxetil Tablets, USP - Rx only - To reduce the development of drug-resistant bacteria and maintain the effectiveness of cefpodoxime proxetil tablets, USP and other antibacterial ...
-
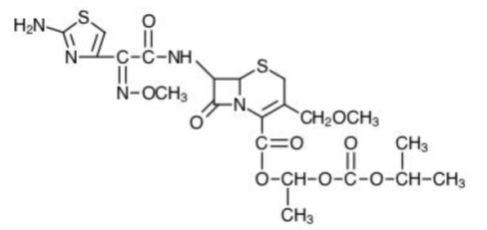
DESCRIPTIONCefpodoxime proxetil is an orally administered, extended spectrum, semi-synthetic antibiotic of the cephalosporin class. The chemical name is (RS)-1(isopropoxycarbonyloxy) ethyl (+)-(6R ...
-
CLINICAL PHARMACOLOGYAbsorption and Excretion - Cefpodoxime proxetil is a prodrug that is absorbed from the gastrointestinal tract and de-esterified to its active metabolite, cefpodoxime. Following oral administration ...
-
INDICATIONS & USAGECefpodoxime proxetil is indicated for the treatment of patients with mild to moderate infections caused by susceptible strains of the designated microorganisms in the conditions listed ...
-
CONTRAINDICATIONSCefpodoxime proxetil is contraindicated in patients with a known allergy to cefpodoxime or to the cephalosporin group of antibiotics.
-
WARNINGSBEFORE THERAPY WITH CEFPODOXIME PROXETIL IS INSTITUTED, CAREFUL INQUIRY SHOULD BE MADE TO DETERMINE WHETHER THE PATIENT HAS HAD PREVIOUS HYPERSENSITIVITY REACTIONS TO CEFPODOXIME, OTHER ...
-
PRECAUTIONSGENERAL - In patients with transient or persistent reduction in urinary output due to renal insufficiency, the total daily dose of cefpodoxime proxetil should be reduced because high and ...
-
ADVERSE REACTIONSClinical Trials - Film-coated Tablets (Multiple dose) In clinical trials using multiple doses of cefpodoxime proxetil film-coated tablets, 4696 patients were treated with the recommended ...
-
OVERDOSAGEIn acute rodent toxicity studies, a single 5 g/kg oral dose produced no adverse effects. In the event of serious toxic reaction from overdosage, hemodialysis or peritoneal dialysis may aid in the ...
-
DOSAGE & ADMINISTRATION(See INDICATIONS AND USAGE for indicated pathogens.) Film-coated Tablets - Cefpodoxime Proxetil Tablets, USP should be administered orally with food to enhance absorption. (See CLINICAL ...
-
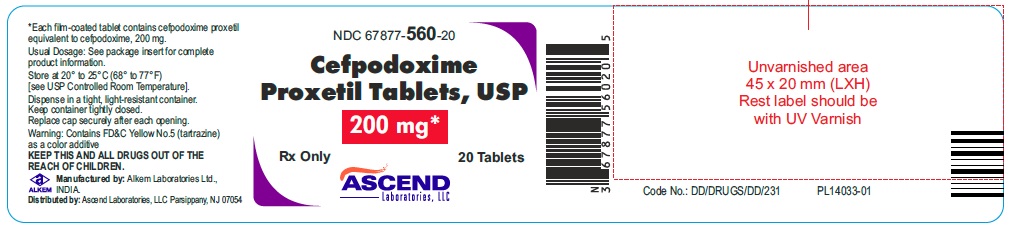
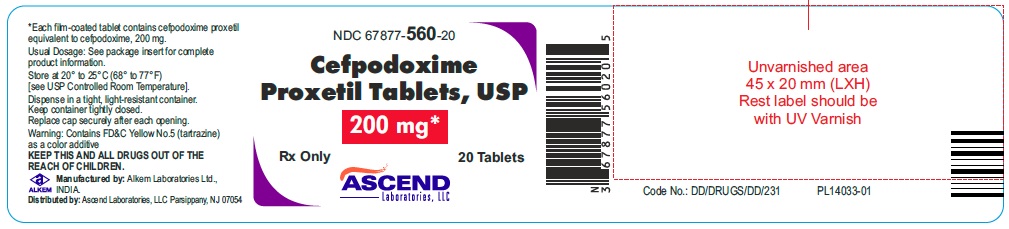
HOW SUPPLIEDCefpodoxime Proxetil Tablets, USP are available in the following strengths (cefpodoxime equivalent), colors, and sizes: 100 mg, (Orange colored, oval shaped, film coated tablets debossed with ...
-
CLINICAL TRIALSCystitis - In two double-blind, 2:1 randomized, comparative trials performed in adults in the United States, cefpodoxime proxetil was compared to other beta-lactam antibiotics. In these studies ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 67877-559-20 - Cefpodoxime Proxetil - Tablets, USP 100 mg* Rx only 20 Tablets - NDC 67877-560-20 - Cefpodoxime Proxetil - Tablets, USP 200 mg* Rx only 20 Tablets
-
INGREDIENTS AND APPEARANCEProduct Information