Label: CEFDINIR powder, for suspension
- NDC Code(s): 67877-547-88, 67877-547-98, 67877-548-88, 67877-548-98
- Packager: Ascend Laboratories, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 29, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONTo reduce the development of drug-resistant bacteria and maintain the effectiveness of cefdinir and other antibacterial drugs, cefdinir should be used only to treat or prevent infections that are ...
-
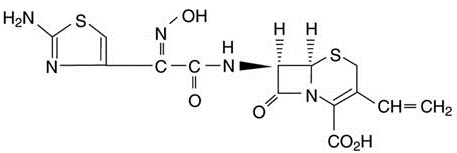
DESCRIPTIONCefdinir for Oral Suspension USP contains the active ingredient cefdinir, an extended-spectrum, semisynthetic cephalosporin, for oral administration. Chemically, cefdinir is [6R-[6α, 7 ...
-
CLINICAL PHARMACOLOGYPharmacokinetics and Drug Metabolism - Absorption - Oral Bioavailability - Maximal plasma cefdinir concentrations occur 2 to 4 hours postdose following capsule or suspension administration ...
-
INDICATIONS & USAGETo reduce the development of drug-resistant bacteria and maintain the effectiveness of cefdinir and other antibacterial drugs, cefdinir should be used only to treat or prevent infections that are ...
-
CONTRAINDICATIONSCefdinir is contraindicated in patients with known allergy to the cephalosporin class of antibiotics.
-
WARNINGSBEFORE THERAPY WITH CEFDINIR IS INSTITUTED, CAREFUL INQUIRY SHOULD BE MADE TO DETERMINE WHETHER THE PATIENT HAS HAD PREVIOUS HYPERSENSITIVITY REACTIONS TO CEFDINIR, OTHER CEPHALOSPORINS ...
-
PRECAUTIONSGENERAL - Prescribing cefdinir in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the ...
-
ADVERSE EVENTSClinical Trials-Cefdinir for Oral Suspension (Pediatric Patients) In clinical trials, 2289 pediatric patients (1783 U.S. and 506 non-U.S.) were treated with the recommended dose of cefdinir ...
-
OVERDOSAGEInformation on cefdinir overdosage in humans is not available. In acute rodent toxicity studies, a single oral 5600- mg/kg dose produced no adverse effects. Toxic signs and symptoms following ...
-
DOSAGE & ADMINISTRATION(see INDICATIONS AND USAGEfor Indicated Pathogens) Powder for Oral Suspension - The recommended dosage and duration of treatment for infections in pediatric patients are described in the ...
-
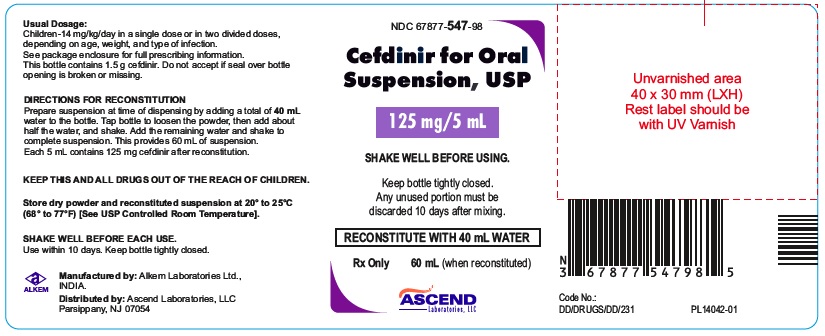
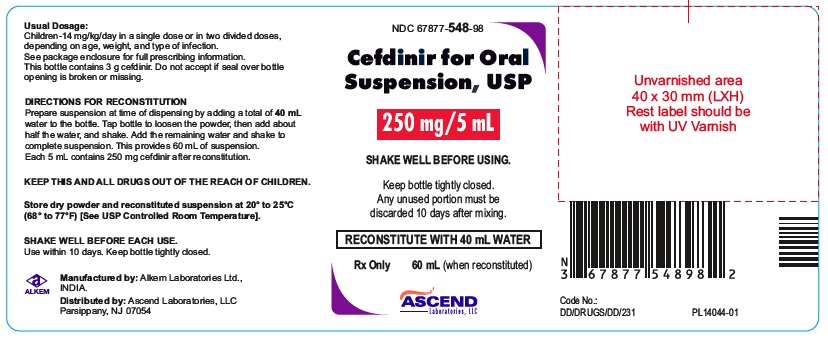
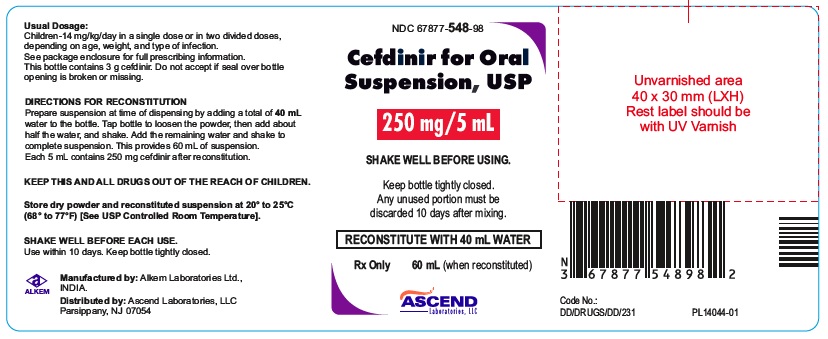
HOW SUPPLIEDCefdinir for Oral Suspension USP is a off white to yellow free flowing powder filled in HDPE bottle that, when reconstituted as directed, contains 125 mg cefdinir/5 mL or 250 mg cefdinir/5 ...
-
CLINICAL STUDIESCommunity-Acquired Bacterial Pneumonia - In a controlled, double-blind study in adults and adolescents conducted in the US, cefdinir BID was compared with cefaclor 500 mg TID. Using strict ...
-
REFERENCESCockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16:31-41. Schwartz GJ, Haycock GB, Edelmann CM, Spitzer A. A simple estimate of glomerular ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL60 mL bottle (Container); NDC 67877-547-98 - Cefdinir for Oral Suspension USP 125 mg/5 mL - Rx Only - 60 mL bottle - 60 mL bottle (Container); NDC 67877-548-98 - Cefdinir for Oral Suspension USP 250 ...
-
INGREDIENTS AND APPEARANCEProduct Information