Label: CEPHALEXIN for suspension
- NDC Code(s): 67877-544-68, 67877-544-88, 67877-545-68, 67877-545-88
- Packager: Ascend Laboratories, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 7, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use CEPHALEXIN FOR ORAL SUSPENSION safely and effectively. See full prescribing information for CEPHALEXIN FOR ORAL SUSPENSION ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS & USAGE1.1 Respiratory Tract Infections - Cephalexin is indicated for the treatment of respiratory tract infections caused by susceptible isolates of Streptococcus pneumoniae and Streptococcus ...
-
2 DOSAGE & ADMINISTRATION2.1 Adults and Pediatric Patients at Least 15 Years of Age - The usual dose of oral cephalexin is 250 mg every 6 hours, but a dose of 500 mg every 12 hours may be administered. Treatment is ...
-
3 DOSAGE FORMS & STRENGTHSCephalexin For Oral Suspension USP - 125 mg/5mL and 250 mg/5mL
-
4 CONTRAINDICATIONSCephalexin is contraindicated in patients with known hypersensitivity to cephalexin or other members of the cephalosporin class of antibacterial drugs.
-
5 WARNINGS AND PRECAUTIONS5.1 Hypersensitivity Reactions - Allergic reactions in the form of rash, urticaria, angioedema, anaphylaxis, erythema multiforme, Stevens-Johnson syndrome, or toxic epidermal necrolysis have been ...
-
6 ADVERSE REACTIONSThe following serious events are described in greater detail in the Warning and Precautions section: • Hypersensitivity reactions [see Warning and Precautions (5.1)] • Clostridium ...
-
7 DRUG INTERACTIONS7.1 Metformin - Administration of cephalexin with metformin results in increased plasma metformin concentrations and decreased renal clearance of metformin. Careful patient monitoring and dose ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Pregnancy Category B - There are no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this ...
-
10 OVERDOSAGESymptoms of oral overdose may include nausea, vomiting, epigastric distress, diarrhea, and hematuria. In the event of an overdose, institute general supportive measures. Forced diuresis ...
-
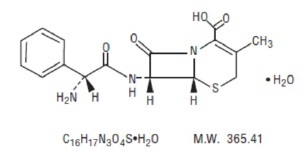
11 DESCRIPTIONCephalexin oral suspension, USP is a semisynthetic cephalosporin antibacterial drug intended for oral administration. It is 7-(D-α- Amino-α-phenylacetamido)-3-methyl-3-cephem-4-carboxylic acid ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Cephalexin is a cephalosporin antibacterial drug [see Microbiology (12.4)]. 12.3 Pharmacokinetics - Absorption: Cephalexin is acid stable and may be given without ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis & Mutagenesis & Impairment Of Fertility - Lifetime studies in animals have not been performed to evaluate the carcinogenic potential of cephalexin. Tests to determine the ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGCephalexin for oral suspension* USP (a strawberry flavored formula) is supplied as follows: 125 mg/5 mL: Bottles of 100 mL (NDC 67877-544-88) Bottles of 200 mL (NDC 67877-544-68) 250 mg/5 ...
-
17 PATIENT COUNSELING INFORMATIONAdvise patients that allergic reactions, including serious allergic reactions, could occur and that serious reactions require immediate treatment. Ask the patient about any previous ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 67877-544-88 - Cephalexin for Oral Suspension, USP 125 mg per 5 mL - when reconstituted according to directions. Usual Pediatric Dose: 25 to 50 mg per kg a day in four divided doses. For more ...
-
INGREDIENTS AND APPEARANCEProduct Information