Label: SOLIFENACIN SUCCINATE tablet, film coated
- NDC Code(s): 67877-527-30, 67877-527-33, 67877-527-38, 67877-527-90, view more
- Packager: Ascend Laboratories, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use SOLIFENACIN SUCCINATE TABLETS safely and effectively. See full prescribing information for SOLIFENACIN SUCCINATE TABLETS ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS & USAGESolifenacin succinate tablets is indicated for the treatment of adults with overactive bladder with symptoms of urge urinary incontinence, urgency, and urinary frequency.
-
2 DOSAGE & ADMINISTRATION2.1 Dosing Information - The recommended oral dose of solifenacin succinate tablets is 5 mg once daily. If the 5 mg dose is well tolerated, the dose may be increased to 10 mg once ...
-
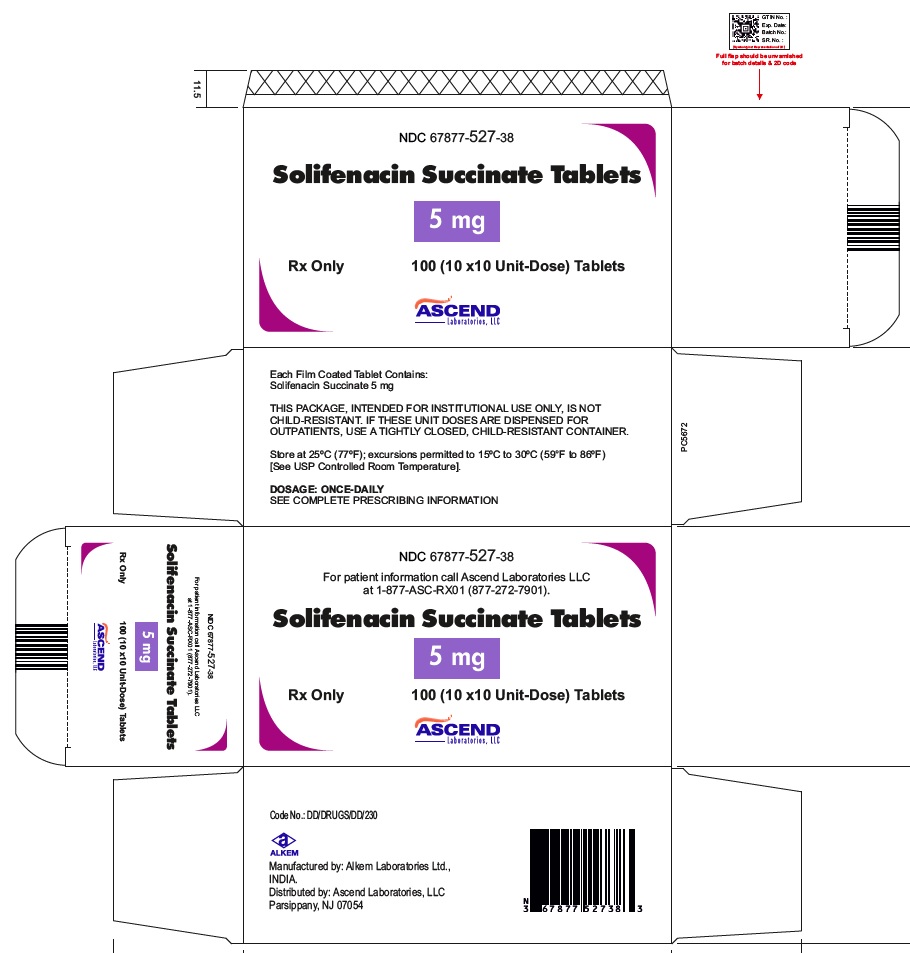
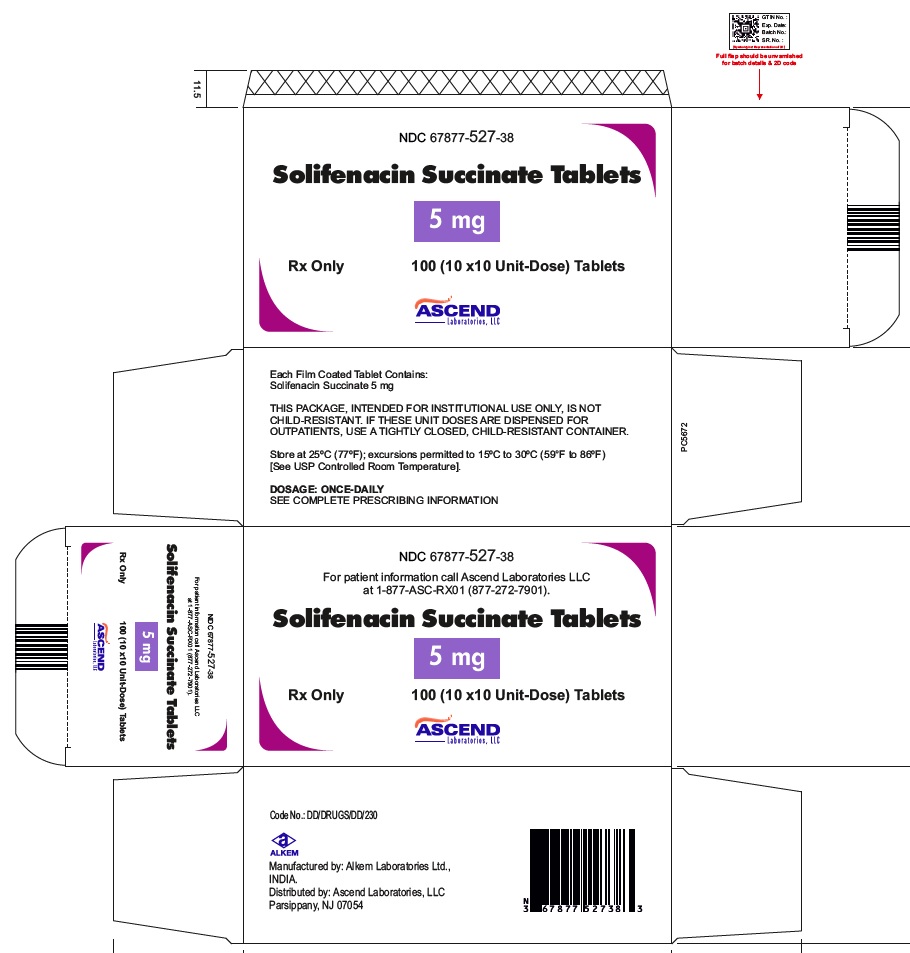
3 DOSAGE FORMS & STRENGTHSThe 5 mg tablets are light yellow, round shaped, film coated debossed with “S5” on one side and plain on other side. The 10 mg tablets are light pink, round shaped, film coated debossed with "S10 ...
-
4 CONTRAINDICATIONSSolifenacin succinate is contraindicated in patients: With urinary retention [see Warnings and Precautions (5.2)], With gastric retention [see Warnings and Precautions (5.3)], With uncontrolled ...
-
5 WARNINGS AND PRECAUTIONS5.1 Angioedema and Anaphylactic Reactions - Angioedema of the face, lips, tongue, and/or larynx have been reported with solifenacin succinate. In some cases, angioedema occurred after the first ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONS7.1 Strong CYP3A4 Inhibitors - Solifenacin is a substrate of CYP3A4. Concomitant use of ketoconazole, a strong CYP3A4 inhibitor, significantly increased the exposure of solifenacin [see Clinical ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no studies with the use of solifenacin succinate in pregnant women to inform a drug-associated risk of major birth defects, miscarriages, or adverse ...
-
10 OVERDOSAGEOverdosage with solifenacin succinate can potentially result in severe antimuscarinic effects and should be treated accordingly. The highest dose ingested in an accidental overdose of solifenacin ...
-
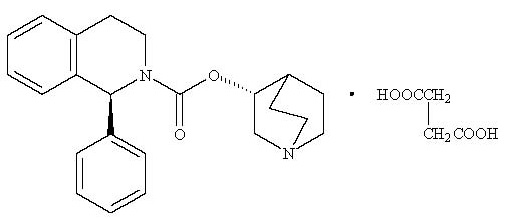
11 DESCRIPTIONSolifenacin succinate is a muscarinic receptor antagonist. Chemically, solifenacin succinate is a butanedioic acid compound with (1S)-(3R)-1-azabicyclo[2.2.2]oct-3-yl ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Solifenacin is a competitive muscarinic receptor antagonist. Muscarinic receptors play an important role in several major cholinergically mediated functions, including ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis & Mutagenesis & Impairment Of Fertility - No increase in tumors was found following the administration of solifenacin succinate to male and female mice for 104 weeks at doses ...
-
14 CLINICAL STUDIESSolifenacin succinate was evaluated in four twelve-week, double-blind, randomized, placebo-controlled, parallel group, multicenter clinical trials for the treatment of overactive bladder in adult ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGSolifenacin succinate tablets are supplied as round, film-coated tablets, available in bottles and unit-dose blister packages as follows: 5 mg tablets: Light yellow, round shaped, film coated ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Angioedema and Anaphylactic Reactions - Inform patients that angioedema and anaphylactic reactions have been ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 67877-527-30 - Solifenacin Succinate Tablets 5 mg - Rx Only - 30 Tablets - NDC 67877-528-90 - Solifenacin Succinate Tablets 10 mg - Rx Only - 90 Tablets - NDC 67877-527-38 - Solifenacin ...
-
INGREDIENTS AND APPEARANCEProduct Information