Label: FINASTERIDE tablet, coated
- NDC Code(s): 67877-455-05, 67877-455-30, 67877-455-34, 67877-455-90, view more
- Packager: Ascend Laboratories, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 24, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use FINASTERIDE TABLETS safely and effectively. See full prescribing information for FINASTERIDE TABLETS. FINASTERIDE tablets for oral ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEFinasteride tablets is indicated for the treatment of male pattern hair loss (androgenetic alopecia) in MEN ONLY. Efficacy in bitemporal recession has not been established. Finasteride tablets ...
-
2 DOSAGE AND ADMINISTRATIONFinasteride tablets may be administered with or without meals. The recommended dose of finasteride tablets, USP 1 mg is one tablet (1 mg) taken once daily. In general, daily use for three ...
-
3 DOSAGE FORMS AND STRENGTHSFinasteride tablets (1 mg) are reddish brown colored, 7 mm round, biconvex, film coated tablets, marked “F1” on one side and plain on other side.
-
4 CONTRAINDICATIONSFinasteride tablets is contraindicated in the following: Pregnancy. Finasteride use is contraindicated in women when they are or may potentially be pregnant. Because of the ability of Type II ...
-
5 WARNINGS AND PRECAUTIONS5.1 Exposure of Women — Risk to Male Fetus - Finasteride tablets is not indicated for use in women. Women should not handle crushed or broken finasteride tablets ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in ...
-
7 DRUG INTERACTIONS7.1 Cytochrome P450-Linked Drug Metabolizing Enzyme System - No drug interactions of clinical importance have been identified. Finasteride does not appear to affect the cytochrome P450-linked ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Pregnancy Category X [see Contraindications (4)]. Finasteride tablets is contraindicated for use in women who are or may become pregnant. Finasteride tablets is a Type ...
-
10 OVERDOSAGEIn clinical studies, single doses of finasteride up to 400 mg and multiple doses of finasteride up to 80 mg/day for three months did not result in adverse reactions. Until further experience is ...
-
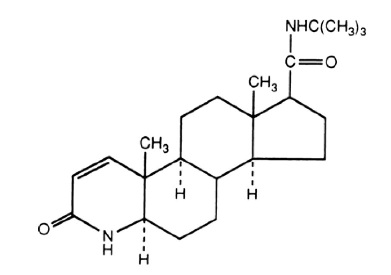
11 DESCRIPTIONFinasteride tablets, USP contain finasteride as the active ingredient. Finasteride, a synthetic ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Finasteride is a competitive and specific inhibitor of Type II 5α-reductase, an intracellular enzyme that converts the androgen testosterone into DHT. Two distinct ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No evidence of a tumorigenic effect was observed in a 24-month study in Sprague-Dawley rats receiving doses of finasteride up to 160 ...
-
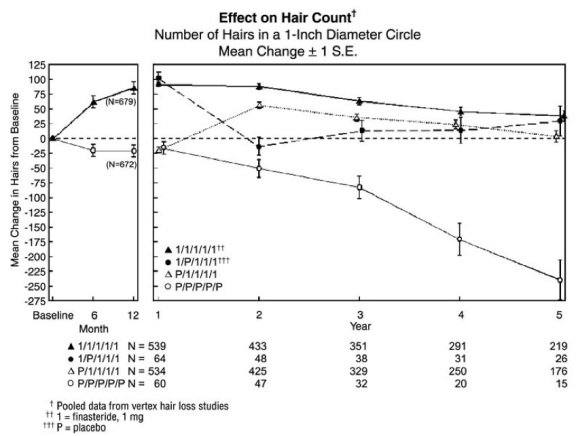
14 CLINICAL STUDIES14.1 Studies in Men - The efficacy of finasteride tablets was demonstrated in men (88% Caucasian) with mild to moderate androgenetic alopecia (male pattern hair loss) between 18 and 41 years ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGFinasteride tablets, USP 1 mg are available as reddish brown colored, 7 mm round, biconvex, film coated tablets, marked “F1” on one side and plain on other side. They are supplied as ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Exposure of Women — Risk to Male ...
-
Patient InformationFinasteride Tablets, USP - (fin NAH steh ride) Finasteride Tablets, USP is for use by MEN ONLY and should NOT be used by women or ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELFinasteride Tablets, USP 1 mg - 90 Tablets Finasteride Tablets, USP 1 mg - 500 Tablets
-
INGREDIENTS AND APPEARANCEProduct Information