Label: NEBIVOLOL tablet
- NDC Code(s): 67877-390-01, 67877-390-30, 67877-390-33, 67877-390-90, view more
- Packager: Ascend Laboratories, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use NEBIVOLOL TABLETS safely and effectively. See full prescribing information for NEBIVOLOL TABLETS. NEBIVOLOL tablets, for oral use ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Hypertension - Nebivolol tablets are indicated for the treatment of hypertension, to lower blood pressure [see Clinical Studies (14.1)]. Nebivolol tablets may be used alone or in combination ...
-
2 DOSAGE AND ADMINISTRATION2.1 Hypertension - The dose of nebivolol must be individualized to the needs of the patient. For most patients, the recommended starting dose is 5 mg once daily, with or without food, as ...
-
3 DOSAGE FORMS AND STRENGTHSNebivolol is available as tablets for oral administration containing nebivolol hydrochloride equivalent to 2.5, 5, 10, and 20 mg of nebivolol. Nebivolol tablets are uncoated round-shaped ...
-
4 CONTRAINDICATIONSNebivolol is contraindicated in the following conditions: Severe bradycardia - Heart block greater than first degree - Patients with cardiogenic shock - Decompensated cardiac failure - Sick sinus ...
-
5 WARNINGS AND PRECAUTIONS5.1 Abrupt Cessation of Therapy - Do not abruptly discontinue nebivolol therapy in patients with coronary artery disease. Severe exacerbation of angina, myocardial infarction and ventricular ...
-
6 ADVERSE REACTIONS6.1 Clinical Studies Experience - Nebivolol has been evaluated for safety in patients with hypertension and in patients with heart failure. The observed adverse reaction profile was consistent ...
-
7 DRUG INTERACTIONS7.1 CYP2D6 Inhibitors - Use caution when nebivolol is co-administered with CYP2D6 inhibitors (quinidine, propafenone, fluoxetine, paroxetine, etc.) [see Clinical Pharmacology (12.5)]. 7.2 ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available data regarding use of nebivolol in pregnant women are insufficient to determine whether there are drug-associated risks of adverse developmental ...
-
10 OVERDOSAGEIn clinical trials and worldwide postmarketing experience there were reports of nebivolol overdose. The most common signs and symptoms associated with nebivolol overdosage are bradycardia and ...
-
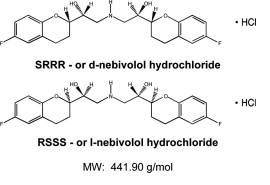
11 DESCRIPTIONThe chemical name for the active ingredient in nebivolol tablets is (1RS, 1’RS)-1, 1’-[(2RS, 2’SR)-bis (6-fluoro-3, 4-dihydro-2H-1-benzopyran-2-yl)]- 2, 2’-iminodiethanol hydrochloride ...
-
12 CLINICAL PHARMACOLOGYNebivolol is a β-adrenergic receptor blocking agent. In extensive metabolizers (most of the population) and at doses less than or equal to 10 mg, nebivolol is preferentially β1 selective. In ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - In a two-year study of nebivolol in mice, a statistically significant increase in the incidence of testicular Leydig cell hyperplasia ...
-
14 CLINICAL STUDIES14.1 Hypertension - The antihypertensive effectiveness of nebivolol as monotherapy has been demonstrated in three randomized, double-blind, multi-center, placebo-controlled trials at doses ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGNebivolol is available as tablets for oral administration containing nebivolol hydrochloride equivalent to 2.5, 5, 10, and 20 mg of nebivolol. Nebivolol tablets are uncoated round-shaped ...
-
17 PATIENT COUNSELING INFORMATIONSee FDA-approved patient labeling (Patient Information). Patient Advice - Advise patients to take nebivolol regularly and continuously, as directed. Nebivolol can be taken with or ...
-
PATIENT INFORMATIONNebivolol (ne-BIV-oh-lol) Tablets - Read the Patient Information that comes with nebivolol tablets before you start taking it and each time you get a refill. There may be new information. This ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNebivolol Tablets 2.5 mg - 30's Count Container Label - Ascend Laboratories LLC - NDC 67877-393-30 - Nebivolol Tablets - 2.5 mg - 30 Tablets - Rx Only - Nebivolol Tablets 5 mg - 90's Count Container ...
-
INGREDIENTS AND APPEARANCEProduct Information