Label: ALISKIREN- aliskiren hemifumarate tablet, film coated
- NDC Code(s): 66993-141-30, 66993-142-30
- Packager: Prasco Laboratories
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application Authorized Generic
Drug Label Information
Updated May 1, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use Aliskiren safely and effectively. See full prescribing information for Aliskiren. Aliskiren tablets, for oral use - Initial U.S ...
-
Table of ContentsTable of Contents
- BOXED WARNING (What is this?)
-
1 INDICATIONS AND USAGE1.1 Hypertension - Aliskiren is indicated for the treatment of hypertension in adults and in pediatric patients weighing 50 kg or greater who are at least 6 years of age and older to lower blood ...
-
2 DOSAGE AND ADMINISTRATION2.1 - Recommended Dosage - In adult patients and in pediatric patients weighing 50 kg or greater who are at least 6 years of age, the recommended starting dose of Aliskiren is 150 mg once ...
-
3 DOSAGE FORMS AND STRENGTHS150 mg light pink biconvex round tablet, imprinted NVR/IL (Side 1/Side 2). 300 mg light red biconvex ovaloid round tablet, imprinted NVR/IU (Side 1/Side 2).
-
4 CONTRAINDICATIONSDo not use Aliskiren with ARBs or ACEIs in patients with diabetes [see Warnings and Precautions (5.2) and Clinical Studies (14.3)]. Aliskiren is contraindicated in patients with known ...
-
5 WARNINGS AND PRECAUTIONS
5.1 Fetal Toxicity - Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - The following serious adverse reactions are discussed in greater detail in other sections of the label: Fetal Toxicity [see Warnings and Precautions ...
-
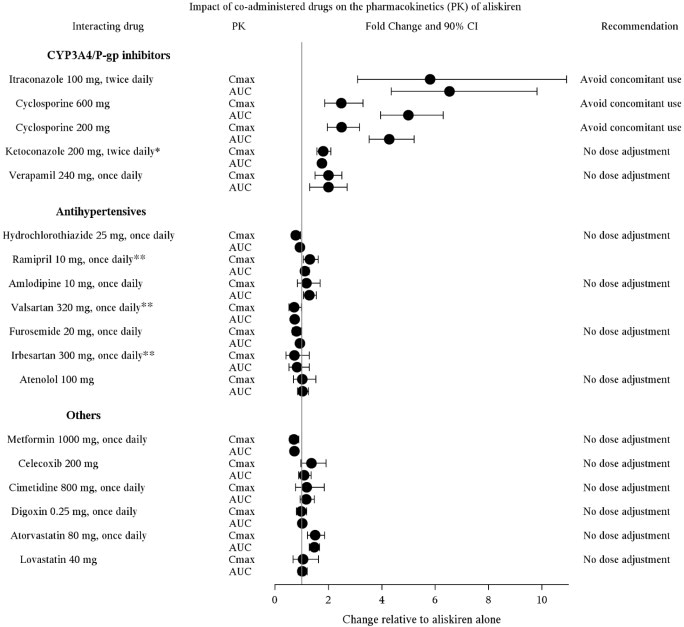
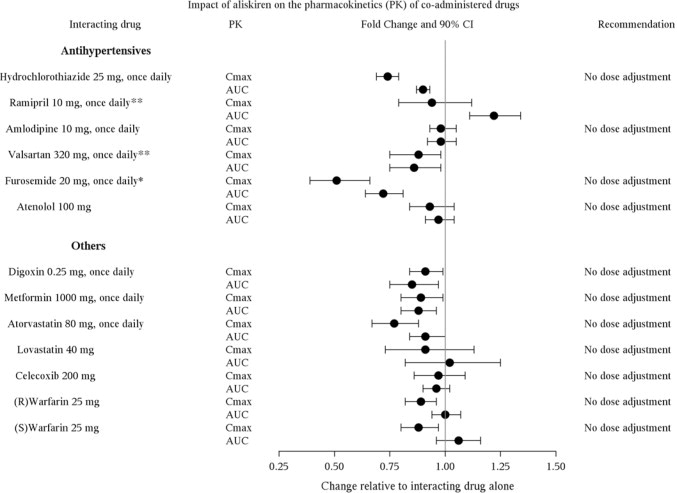
7 DRUG INTERACTIONSCyclosporine: Avoid coadministration of cyclosporine with aliskiren [see Warnings and Precautions (5.7) and Clinical Pharmacology (12.3)]. Itraconazole: Avoid coadministration of itraconazole ...
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Risk Summary - Aliskiren can cause fetal harm when administered to a pregnant woman. Use of drugs that act on the renin-angiotensin system during the second and third ...
-
10 OVERDOSAGE
Limited data are available related to overdosage in humans. The most likely manifestation of overdosage would be hypotension. If symptomatic hypotension occurs, supportive treatment should be ...
-
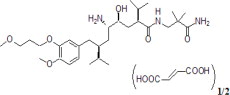
11 DESCRIPTIONAliskiren contains aliskiren hemifumarate, adirect renin inhibitor. Aliskiren hemifumarate is chemically described as (2S,4S,5S,7S)-N-(2-carbamoyl-2-methylpropyl)-5-amino-4-hydroxy ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - Renin is secreted by the kidney in response to decreases in blood volume and renal perfusion. Renin cleaves angiotensinogen to form the inactive decapeptide ...
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenic potential was assessed in a 2-year rat study and a 6-month transgenic (rasH2) mouse study with aliskiren hemifumarate at ...
-
14 CLINICAL STUDIES
14.1 Aliskiren Monotherapy - The antihypertensive effects of Aliskiren have been demonstrated in 6 randomized, double-blind, placebo-controlled 8- week clinical trials in patients with ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGAliskiren tablets are supplied as a light-pink, biconvex round tablet containing 150 mg of aliskiren, and as a light-red biconvex ovaloid tablet containing 300 mg of aliskiren. Tablets are ...
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information) and Instructions for Use. Information for Patients - Pregnancy: Advise female patients of ...
-
PATIENT PACKAGE INSERTPATIENT INFORMATION - Aliskiren Tablets - What is the most important information I should know about Aliskiren? Aliskiren can cause harm or death to your unborn baby. Talk to your doctor ...
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel - Package Label – 150 mg - NDC 66993-141-30 - 30 Tablets - Rx only - PRASCO - Aliskiren - Tablets - 150 mg
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel - Package Label – 300 mg - NDC 66993-142-30 - 30 Tablets - Rx only - PRASCO - Aliskiren - Tablets - 300 mg
-
INGREDIENTS AND APPEARANCEProduct Information