Label: FLUTICASONE PROPIONATE AND SALMETEROL HFA- fluticasone propionate and salmeterol xinafoate aerosol, metered
- NDC Code(s): 66993-086-96, 66993-087-96, 66993-088-96
- Packager: Prasco Laboratories
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application Authorized Generic
Drug Label Information
Updated May 29, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use Fluticasone Propionate and Salmeterol HFA safely and effectively. See full prescribing information for Fluticasone Propionate and ...These highlights do not include all the information needed to use Fluticasone Propionate and Salmeterol HFA safely and effectively. See full prescribing information for Fluticasone Propionate and Salmeterol HFA.
Fluticasone Propionate and Salmeterol HFA inhalation aerosol, for oral inhalation use
Initial U.S. Approval: 2000INDICATIONS AND USAGE
Fluticasone Propionate and Salmeterol HFA is a combination of fluticasone propionate, an inhaled corticosteroid, and salmeterol, a long-acting beta2‑adrenergic agonist (LABA), indicated for treatment of asthma in adult and adolescent patients aged 12 years and older. (1)
Limitations of use: Not indicated for relief of acute bronchospasm. (1)
DOSAGE AND ADMINISTRATION
- •
- For oral inhalation only. (2.1)
- •
- Adult and adolescent patients aged 12 years and older: 2 inhalations of Fluticasone Propionate and Salmeterol HFA 45 mcg/21 mcg, Fluticasone Propionate and Salmeterol HFA 115 mcg/21 mcg, or Fluticasone Propionate and Salmeterol HFA 230 mcg/21 mcg twice daily. (2.2)
- •
- Starting dosage is based on asthma severity. (2.2)
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- •
- LABA monotherapy increases the risk of serious asthma-related events. (5.1)
- •
- Do not initiate in acutely deteriorating asthma or to treat acute symptoms. (5.2)
- •
- Do not use in combination with an additional medicine containing a LABA because of risk of overdose. (5.3)
- •
- Candida albicans infection of the mouth and pharynx may occur. Monitor patients periodically. Advise the patient to rinse his/her mouth with water without swallowing after inhalation to help reduce the risk. (5.4)
- •
- Increased risk of pneumonia in patients with COPD. Monitor patients for signs and symptoms of pneumonia. (5.5)
- •
- Potential worsening of infections (e.g., existing tuberculosis; fungal, bacterial, viral, or parasitic infections; ocular herpes simplex). Use with caution in patients with these infections. More serious or even fatal course of chickenpox or measles can occur in susceptible patients. (5.6)
- •
- Risk of impaired adrenal function when transferring from systemic corticosteroids. Taper patients slowly from systemic corticosteroids if transferring to Fluticasone Propionate and Salmeterol HFA. (5.7)
- •
- Hypercorticism and adrenal suppression may occur with very high dosages or at the regular dosage in susceptible individuals. If such changes occur, discontinue Fluticasone Propionate and Salmeterol HFA slowly. (5.8)
- •
- If paradoxical bronchospasm occurs, discontinue Fluticasone Propionate and Salmeterol HFA and institute alternative therapy. (5.10)
- •
- Use with caution in patients with cardiovascular or central nervous system disorders because of beta-adrenergic stimulation. (5.12)
- •
- Assess for decrease in bone mineral density initially and periodically thereafter. (5.13)
- •
- Monitor growth of pediatric patients. (5.14)
- •
- Glaucoma and cataracts may occur with long-term use of inhaled corticosteroids. Consider referral to an ophthalmologist in patients who develop ocular symptoms or use Fluticasone Propionate and Salmeterol HFA long term. (5.15)
- •
- Be alert to eosinophilic conditions, hypokalemia, and hyperglycemia. (5.16, 5.18)
- •
- Use with caution in patients with convulsive disorders, thyrotoxicosis, diabetes mellitus, and ketoacidosis. (5.17)
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥3%) include: upper respiratory tract infection or inflammation, throat irritation, dysphonia, headache, dizziness, nausea and vomiting. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Prasco Laboratories at 1-866-525-0688 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- •
- Strong cytochrome P450 3A4 inhibitors (e.g., ritonavir, ketoconazole): Use not recommended. May increase risk of systemic corticosteroid and cardiovascular effects. (7.1)
- •
- Monoamine oxidase inhibitors and tricyclic antidepressants: Use with extreme caution. May potentiate effect of salmeterol on vascular system. (7.2)
- •
- Beta-blockers: Use with caution. May block bronchodilatory effects of beta-agonists and produce severe bronchospasm. (7.3)
- •
- Diuretics: Use with caution. Electrocardiographic changes and/or hypokalemia associated with non–potassium-sparing diuretics may worsen with concomitant beta-agonists. (7.4)
USE IN SPECIFIC POPULATIONS
Hepatic impairment: Monitor patients for signs of increased drug exposure. (8.6)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 5/2024
Close -
Table of ContentsTable of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Administration Information
2.2 Recommended Dosage
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Serious Asthma-Related Events – Hospitalizations, Intubations, Death
5.2 Deterioration of Disease and Acute Episodes
5.3 Avoid Excessive Use of Fluticasone Propionate and Salmeterol HFA and Avoid Use with Other Long-acting Beta2-agonists
5.4 Oropharyngeal Candidiasis
5.5 Pneumonia
5.6 Immunosuppression and Risk of Infections
5.7 Transferring Patients from Systemic Corticosteroid Therapy
5.8 Hypercorticism and Adrenal Suppression
5.9 Drug Interactions with Strong Cytochrome P450 3A4 Inhibitors
5.10 Paradoxical Bronchospasm and Upper Airway Symptoms
5.11 Hypersensitivity Reactions, including Anaphylaxis
5.12 Cardiovascular and Central Nervous System Effects
5.13 Reduction in Bone Mineral Density
5.14 Effect on Growth
5.15 Glaucoma and Cataracts
5.16 Eosinophilic Conditions and Churg-Strauss Syndrome
5.17 Coexisting Conditions
5.18 Hypokalemia and Hyperglycemia
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Inhibitors of Cytochrome P450 3A4
7.2 Monoamine Oxidase Inhibitors and Tricyclic Antidepressants
7.3 Beta-adrenergic Receptor Blocking Agents
7.4 Non–Potassium-Sparing Diuretics
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
8.7 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Trials Comparing Fluticasone Propionate and Salmeterol HFA with Fluticasone Propionate Alone or Salmeterol Alone
14.2 One-Year Safety Trial
14.3 Onset of Action and Progression of Improvement in Control
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGEFluticasone Propionate and Salmeterol HFA is indicated for treatment of asthma in adult and adolescent patients aged 12 years and older. Fluticasone Propionate and Salmeterol HFA should be used ...
Fluticasone Propionate and Salmeterol HFA is indicated for treatment of asthma in adult and adolescent patients aged 12 years and older. Fluticasone Propionate and Salmeterol HFA should be used for patients not adequately controlled on a long-term asthma control medication such as an inhaled corticosteroid (ICS) or whose disease warrants initiation of treatment with both an ICS and long-acting beta2-adrenergic agonist (LABA).
Limitations of Use
Fluticasone Propionate and Salmeterol HFA is not indicated for the relief of acute bronchospasm.
Close -
2 DOSAGE AND ADMINISTRATION2.1 Administration Information - Fluticasone Propionate and Salmeterol HFA should be administered by the orally inhaled route only. After inhalation, rinse mouth with water without swallowing to ...
2.1 Administration Information
Fluticasone Propionate and Salmeterol HFA should be administered by the orally inhaled route only. After inhalation, rinse mouth with water without swallowing to help reduce the risk of oropharyngeal candidiasis.
Priming
Prime Fluticasone Propionate and Salmeterol HFA before using for the first time by releasing 4 sprays into the air away from the face, shaking well for 5 seconds before each spray. In cases where the inhaler has not been used for more than 4 weeks or when it has been dropped, prime the inhaler again by releasing 2 sprays into the air away from the face, shaking well for 5 seconds before each spray. Avoid spraying in eyes.
Close2.2 Recommended Dosage
Adult and adolescent patients aged 12 years and older: 2 oral inhalations twice daily, approximately 12 hours apart.
The maximum recommended dosage is 2 inhalations of Fluticasone Propionate and Salmeterol HFA 230 mcg/21 mcg twice daily.
General Dosing Recommendation
When choosing the starting dosage strength of Fluticasone Propionate and Salmeterol HFA, consider the patients’ disease severity, based on their previous asthma therapy, including the ICS dosage, as well as the patients’ current control of asthma symptoms and risk of future exacerbation.
If asthma symptoms arise in the period between doses, an inhaled, short-acting beta2-agonist should be used for immediate relief.
Improvement in asthma control following inhaled administration of Fluticasone Propionate and Salmeterol HFA can occur within 30 minutes of beginning treatment, although maximum benefit may not be achieved for 1 week or longer after starting treatment. Individual patients will experience a variable time to onset and degree of symptom relief.
For patients who do not respond adequately to the starting dosage after 2 weeks of therapy, replacing the current strength of Fluticasone Propionate and Salmeterol HFA with a higher strength may provide additional improvement in asthma control.
If a previously effective dosage regimen fails to provide adequate improvement in asthma control, the therapeutic regimen should be reevaluated and additional therapeutic options (e.g., replacing the current strength of Fluticasone Propionate and Salmeterol HFA with a higher strength, adding additional ICS, initiating oral corticosteroids) should be considered.
More frequent administration or a greater number of inhalations (more than 2 inhalations twice daily) of the prescribed strength of Fluticasone Propionate and Salmeterol HFA is not recommended as some patients are more likely to experience adverse effects with higher doses of salmeterol. Patients using Fluticasone Propionate and Salmeterol HFA should not use additional LABA for any reason. [See Warnings and Precautions (5.3, 5.12).]
-
3 DOSAGE FORMS AND STRENGTHSInhalation aerosol: purple plastic inhaler with a light purple cap containing a pressurized metered-dose aerosol canister containing 120 metered inhalations and fitted with a counter. • 45 mcg ...
Inhalation aerosol: purple plastic inhaler with a light purple cap containing a pressurized metered-dose aerosol canister containing 120 metered inhalations and fitted with a counter.
- •
- 45 mcg fluticasone propionate/21 mcg salmeterol from the mouthpiece per actuation
- •
- 115 mcg fluticasone propionate/21 mcg salmeterol from the mouthpiece per actuation
- •
- 230 mcg fluticasone propionate/21 mcg salmeterol from the mouthpiece per actuation
-
4 CONTRAINDICATIONSFluticasone Propionate and Salmeterol HFA is contraindicated in the following conditions: • Primary treatment of status asthmaticus or other acute episodes of asthma where intensive measures are ...
Fluticasone Propionate and Salmeterol HFA is contraindicated in the following conditions:
- •
- Primary treatment of status asthmaticus or other acute episodes of asthma where intensive measures are required [see Warnings and Precautions (5.2)].
- •
- Hypersensitivity to any of the ingredients [see Warnings and Precautions (5.11), Adverse Reactions (6.2), Description (11)].
-
5 WARNINGS AND PRECAUTIONS5.1 Serious Asthma-Related Events – Hospitalizations, Intubations, Death - Use of LABA as monotherapy (without ICS) for asthma is associated with an increased risk of asthma-related death [see ...
5.1 Serious Asthma-Related Events – Hospitalizations, Intubations, Death
Use of LABA as monotherapy (without ICS) for asthma is associated with an increased risk of asthma-related death [see Salmeterol Multicenter Asthma Research Trial (SMART)]. Available data from controlled clinical trials also suggest that use of LABA as monotherapy increases the risk of asthma-related hospitalization in pediatric and adolescent patients. These findings are considered a class effect of LABA monotherapy. When LABA are used in fixed-dose combination with ICS, data from large clinical trials do not show a significant increase in the risk of serious asthma-related events (hospitalizations, intubations, death) compared with ICS alone (see Serious Asthma-Related Events with Inhaled Corticosteroid/Long-acting Beta2-adrenergic Agonists).
Serious Asthma-Related Events with Inhaled Corticosteroid/Long-acting Beta2-adrenergic Agonists
Four (4) large, 26-week, randomized, double-blind, active-controlled clinical safety trials were conducted to evaluate the risk of serious asthma-related events when LABA were used in fixed‑dose combination with ICS compared with ICS alone in subjects with asthma. Three (3) trials included adult and adolescent subjects aged 12 years and older: 1 trial compared fluticasone propionate/salmeterol inhalation powder with fluticasone propionate inhalation powder, 1 trial compared mometasone furoate/formoterol with mometasone furoate, and 1 trial compared budesonide/formoterol with budesonide. The fourth trial included pediatric subjects aged 4 to 11 years and compared fluticasone propionate/salmeterol inhalation powder with fluticasone propionate inhalation powder. The primary safety endpoint for all 4 trials was serious asthma-related events (hospitalizations, intubations, death). A blinded adjudication committee determined whether events were asthma related.
The 3 adult and adolescent trials were designed to rule out a risk margin of 2.0, and the pediatric trial was designed to rule out a risk margin of 2.7. Each individual trial met its pre-specified objective and demonstrated non-inferiority of ICS/LABA to ICS alone. A meta-analysis of the 3 adult and adolescent trials did not show a significant increase in risk of a serious asthma-related event with ICS/LABA fixed-dose combination compared with ICS alone (Table 1). These trials were not designed to rule out all risk for serious asthma-related events with ICS/LABA compared with ICS.
Table 1. Meta-analysis of Serious Asthma-Related Events in Subjects with Asthma Aged 12 Years and Older ICS = Inhaled Corticosteroid, LABA = Long-acting Beta2-adrenergic Agonist.
a Randomized subjects who had taken at least 1 dose of study drug. Planned treatment used for analysis.
b Estimated using a Cox proportional hazards model for time to first event with baseline hazards stratified by each of the 3 trials.
c Number of subjects with event that occurred within 6 months after the first use of study drug or 7 days after the last date of study drug, whichever date was later. Subjects can have one or more events, but only the first event was counted for analysis. A single, blinded, independent adjudication committee determined whether events were asthma related.ICS/LABA
(n = 17,537)a
ICS
(n = 17,552)a
ICS/LABA vs. ICS
Hazard Ratio
(95% CI)b
Serious asthma-related eventc
116
105
1.10 (0.85, 1.44)
Asthma-related death
2
0
Asthma-related intubation (endotracheal)
1
2
Asthma-related hospitalization (≥24-hour stay)
115
105
The pediatric safety trial included 6,208 pediatric subjects aged 4 to 11 years who received ICS/LABA (fluticasone propionate/salmeterol inhalation powder) or ICS (fluticasone propionate inhalation powder). In this trial, 27/3,107 (0.9%) subjects randomized to ICS/LABA and 21/3,101 (0.7%) subjects randomized to ICS experienced a serious asthma-related event. There were no asthma-related deaths or intubations. ICS/LABA did not show a significantly increased risk of a serious asthma-related event compared with ICS based on the pre-specified risk margin (2.7), with an estimated hazard ratio of time to first event of 1.29 (95% CI: 0.73, 2.27).
Salmeterol Multicenter Asthma Research Trial (SMART)
A 28-week, placebo-controlled, U.S. trial that compared the safety of salmeterol with placebo, each added to usual asthma therapy, showed an increase in asthma-related deaths in subjects receiving salmeterol (13/13,176 in subjects treated with salmeterol versus 3/13,179 in subjects treated with placebo; relative risk: 4.37 [95% CI: 1.25, 15.34]). Use of background ICS was not required in SMART. The increased risk of asthma‑related death is considered a class effect of LABA monotherapy.
5.2 Deterioration of Disease and Acute Episodes
Fluticasone Propionate and Salmeterol HFA should not be initiated in patients during rapidly deteriorating or potentially life-threatening episodes of asthma. Fluticasone propionate and salmeterol HFA has not been studied in subjects with acutely deteriorating asthma. The initiation of Fluticasone Propionate and Salmeterol HFA in this setting is not appropriate.
Serious acute respiratory events, including fatalities, have been reported when salmeterol, a component of Fluticasone Propionate and Salmeterol HFA, has been initiated in patients with significantly worsening or acutely deteriorating asthma. In most cases, these have occurred in patients with severe asthma (e.g., patients with a history of corticosteroid dependence, low pulmonary function, intubation, mechanical ventilation, frequent hospitalizations, previous life-threatening acute asthma exacerbations) and in some patients with acutely deteriorating asthma (e.g., patients with significantly increasing symptoms; increasing need for inhaled, short-acting beta2-agonists; decreasing response to usual medications; increasing need for systemic corticosteroids; recent emergency room visits; deteriorating lung function). However, these events have occurred in a few patients with less severe asthma as well. It was not possible from these reports to determine whether salmeterol contributed to these events.
Increasing use of inhaled, short-acting beta2-agonists is a marker of deteriorating asthma. In this situation, the patient requires immediate reevaluation with reassessment of the treatment regimen, giving special consideration to the possible need for replacing the current strength of Fluticasone Propionate and Salmeterol HFA with a higher strength, adding additional ICS, or initiating systemic corticosteroids. Patients should not use more than 2 inhalations twice daily of Fluticasone Propionate and Salmeterol HFA.
Fluticasone Propionate and Salmeterol HFA should not be used for the relief of acute symptoms, i.e., as rescue therapy for the treatment of acute episodes of bronchospasm. Fluticasone propionate and salmeterol HFA has not been studied in the relief of acute symptoms and extra doses should not be used for that purpose. Acute symptoms should be treated with an inhaled, short-acting beta2-agonist.
When beginning treatment with Fluticasone Propionate and Salmeterol HFA, patients who have been taking oral or inhaled, short-acting beta2-agonists on a regular basis (e.g., 4 times a day) should be instructed to discontinue the regular use of these drugs.
5.3 Avoid Excessive Use of Fluticasone Propionate and Salmeterol HFA and Avoid Use with Other Long-acting Beta2-agonists
Fluticasone Propionate and Salmeterol HFA should not be used more often than recommended, at higher doses than recommended, or in conjunction with other medicines containing LABA, as an overdose may result. Clinically significant cardiovascular effects and fatalities have been reported in association with excessive use of inhaled sympathomimetic drugs. Patients using Fluticasone Propionate and Salmeterol HFA should not use another medicine containing a LABA (e.g., salmeterol, formoterol fumarate, arformoterol tartrate, indacaterol) for any reason.
5.4 Oropharyngeal Candidiasis
In clinical trials, the development of localized infections of the mouth and pharynx with Candida albicans has occurred in subjects treated with fluticasone propionate and salmeterol HFA. When such an infection develops, it should be treated with appropriate local or systemic (i.e., oral) antifungal therapy while treatment with Fluticasone Propionate and Salmeterol HFA continues, but at times therapy with Fluticasone Propionate and Salmeterol HFA may need to be interrupted. Advise the patient to rinse his/her mouth with water without swallowing following inhalation to help reduce the risk of oropharyngeal candidiasis.
5.5 Pneumonia
Lower respiratory tract infections, including pneumonia, have been reported in patients with chronic obstructive pulmonary disease (COPD) following the inhaled administration of corticosteroids, including fluticasone propionate and ADVAIR DISKUS (fluticasone propionate and salmeterol inhalation powder). In 2 replicate 1-year trials in 1,579 subjects with COPD, there was a higher incidence of pneumonia reported in subjects receiving ADVAIR DISKUS 250 mcg/50 mcg (7%) than in those receiving salmeterol 50 mcg (3%). The incidence of pneumonia in the subjects treated with ADVAIR DISKUS was higher in subjects older than 65 years (9%) compared with the incidence in subjects younger than 65 years (4%).
In a 3-year trial in 6,184 subjects with COPD, there was a higher incidence of pneumonia reported in subjects receiving ADVAIR DISKUS 500 mcg/50 mcg compared with placebo (16% with ADVAIR DISKUS 500 mcg/50 mcg, 14% with fluticasone propionate 500 mcg, 11% with salmeterol 50 mcg, and 9% with placebo). Similar to what was seen in the 1-year trials with ADVAIR DISKUS 250 mcg/50 mcg, the incidence of pneumonia was higher in subjects older than 65 years (18% with ADVAIR DISKUS 500 mcg/50 mcg versus 10% with placebo) compared with subjects younger than 65 years (14% with ADVAIR DISKUS 500 mcg/50 mcg versus 8% with placebo).
5.6 Immunosuppression and Risk of Infections
Persons who are using drugs that suppress the immune system are more susceptible to infections than healthy individuals. Chickenpox and measles, for example, can have a more serious or even fatal course in susceptible children or adults using corticosteroids. In such children or adults who have not had these diseases or been properly immunized, particular care should be taken to avoid exposure. How the dose, route, and duration of corticosteroid administration affect the risk of developing a disseminated infection is not known. The contribution of the underlying disease and/or prior corticosteroid treatment to the risk is also not known. If a patient is exposed to chickenpox, prophylaxis with varicella zoster immune globulin (VZIG) may be indicated. If a patient is exposed to measles, prophylaxis with pooled intramuscular immunoglobulin (IG) may be indicated. (See the respective package inserts for complete VZIG and IG prescribing information.) If chickenpox develops, treatment with antiviral agents may be considered.
ICS should be used with caution, if at all, in patients with active or quiescent tuberculosis infections of the respiratory tract; systemic fungal, bacterial, viral, or parasitic infections; or ocular herpes simplex.
5.7 Transferring Patients from Systemic Corticosteroid Therapy
HPA Suppression/Adrenal Insufficiency
Particular care is needed for patients who have been transferred from systemically active corticosteroids to ICS because deaths due to adrenal insufficiency have occurred in patients with asthma during and after transfer from systemic corticosteroids to less systemically available ICS. After withdrawal from systemic corticosteroids, a number of months are required for recovery of hypothalamic-pituitary-adrenal (HPA) function.
Patients who have been previously maintained on 20 mg or more of prednisone (or its equivalent) may be most susceptible, particularly when their systemic corticosteroids have been almost completely withdrawn. During this period of HPA suppression, patients may exhibit signs and symptoms of adrenal insufficiency when exposed to trauma, surgery, or infection (particularly gastroenteritis) or other conditions associated with severe electrolyte loss. Although Fluticasone Propionate and Salmeterol HFA may control asthma symptoms during these episodes, in recommended doses it supplies less than normal physiological amounts of glucocorticoid systemically and does NOT provide the mineralocorticoid activity that is necessary for coping with these emergencies.
During periods of stress or a severe asthma attack, patients who have been withdrawn from systemic corticosteroids should be instructed to resume oral corticosteroids (in large doses) immediately and to contact their physicians for further instruction. These patients should also be instructed to carry a warning card indicating that they may need supplementary systemic corticosteroids during periods of stress or a severe asthma attack.
Patients requiring oral corticosteroids should be weaned slowly from systemic corticosteroid use after transferring to Fluticasone Propionate and Salmeterol HFA. Prednisone reduction can be accomplished by reducing the daily prednisone dose by 2.5 mg on a weekly basis during therapy with Fluticasone Propionate and Salmeterol HFA. Lung function (mean forced expiratory volume in 1 second [FEV1] or morning peak expiratory flow [AM PEF]), beta-agonist use, and asthma symptoms should be carefully monitored during withdrawal of oral corticosteroids. In addition, patients should be observed for signs and symptoms of adrenal insufficiency, such as fatigue, lassitude, weakness, nausea and vomiting, and hypotension.
Unmasking of Allergic Conditions Previously Suppressed by Systemic Corticosteroids
Transfer of patients from systemic corticosteroid therapy to Fluticasone Propionate and Salmeterol HFA may unmask allergic conditions previously suppressed by the systemic corticosteroid therapy (e.g., rhinitis, conjunctivitis, eczema, arthritis, eosinophilic conditions).
Corticosteroid Withdrawal Symptoms
During withdrawal from oral corticosteroids, some patients may experience symptoms of systemically active corticosteroid withdrawal (e.g., joint and/or muscular pain, lassitude, depression) despite maintenance or even improvement of respiratory function.
5.8 Hypercorticism and Adrenal Suppression
Fluticasone propionate, a component of Fluticasone Propionate and Salmeterol HFA, will often help control asthma symptoms with less suppression of HPA function than therapeutically equivalent oral doses of prednisone. Since fluticasone propionate is absorbed into the circulation and can be systemically active at higher doses, the beneficial effects of Fluticasone Propionate and Salmeterol HFA in minimizing HPA dysfunction may be expected only when recommended dosages are not exceeded and individual patients are titrated to the lowest effective dose. A relationship between plasma levels of fluticasone propionate and inhibitory effects on stimulated cortisol production has been shown after 4 weeks of treatment with fluticasone propionate inhalation aerosol. Since individual sensitivity to effects on cortisol production exists, physicians should consider this information when prescribing Fluticasone Propionate and Salmeterol HFA.
Because of the possibility of significant systemic absorption of ICS in sensitive patients, patients treated with Fluticasone Propionate and Salmeterol HFA should be observed carefully for any evidence of systemic corticosteroid effects. Particular care should be taken in observing patients postoperatively or during periods of stress for evidence of inadequate adrenal response.
It is possible that systemic corticosteroid effects such as hypercorticism and adrenal suppression (including adrenal crisis) may appear in a small number of patients who are sensitive to these effects. If such effects occur, Fluticasone Propionate and Salmeterol HFA should be reduced slowly, consistent with accepted procedures for reducing systemic corticosteroids, and other treatments for management of asthma symptoms should be considered.
5.9 Drug Interactions with Strong Cytochrome P450 3A4 Inhibitors
The use of strong cytochrome P450 3A4 (CYP3A4) inhibitors (e.g., ritonavir, atazanavir, clarithromycin, indinavir, itraconazole, nefazodone, nelfinavir, saquinavir, ketoconazole, telithromycin) with Fluticasone Propionate and Salmeterol HFA is not recommended because increased systemic corticosteroid and increased cardiovascular adverse effects may occur [see Drug Interactions (7.1), Clinical Pharmacology (12.3)].
5.10 Paradoxical Bronchospasm and Upper Airway Symptoms
As with other inhaled medicines, Fluticasone Propionate and Salmeterol HFA can produce paradoxical bronchospasm, which may be life threatening. If paradoxical bronchospasm occurs following dosing with Fluticasone Propionate and Salmeterol HFA, it should be treated immediately with an inhaled, short-acting bronchodilator; Fluticasone Propionate and Salmeterol HFA should be discontinued immediately; and alternative therapy should be instituted. Upper airway symptoms of laryngeal spasm, irritation, or swelling, such as stridor and choking, have been reported in patients receiving fluticasone propionate and salmeterol HFA.
5.11 Hypersensitivity Reactions, including Anaphylaxis
Immediate hypersensitivity reactions (e.g., urticaria, angioedema, rash, bronchospasm, hypotension), including anaphylaxis, may occur after administration of Fluticasone Propionate and Salmeterol HFA [see Contraindications (4)].
5.12 Cardiovascular and Central Nervous System Effects
Excessive beta-adrenergic stimulation has been associated with seizures, angina, hypertension or hypotension, tachycardia with rates up to 200 beats/min, arrhythmias, nervousness, headache, tremor, palpitation, nausea, dizziness, fatigue, malaise, and insomnia [see Overdosage (10)]. Therefore, Fluticasone Propionate and Salmeterol HFA, like all products containing sympathomimetic amines, should be used with caution in patients with cardiovascular disorders, especially coronary insufficiency, cardiac arrhythmias, and hypertension.
Salmeterol, a component of Fluticasone Propionate and Salmeterol HFA, can produce a clinically significant cardiovascular effect in some patients as measured by pulse rate, blood pressure, and/or symptoms. Although such effects are uncommon after administration of salmeterol at recommended doses, if they occur, the drug may need to be discontinued. In addition, beta-agonists have been reported to produce electrocardiogram (ECG) changes, such as flattening of the T wave, prolongation of the QTc interval, and ST segment depression. The clinical significance of these findings is unknown. Large doses of inhaled or oral salmeterol (12 to 20 times the recommended dose) have been associated with clinically significant prolongation of the QTc interval, which has the potential for producing ventricular arrhythmias. Fatalities have been reported in association with excessive use of inhaled sympathomimetic drugs.
5.13 Reduction in Bone Mineral Density
Decreases in bone mineral density (BMD) have been observed with long-term administration of products containing ICS. The clinical significance of small changes in BMD with regard to long-term consequences such as fracture is unknown. Patients with major risk factors for decreased bone mineral content, such as prolonged immobilization, family history of osteoporosis, postmenopausal status, tobacco use, advanced age, poor nutrition, or chronic use of drugs that can reduce bone mass (e.g., anticonvulsants, oral corticosteroids), should be monitored and treated with established standards of care.
2-Year Fluticasone Propionate Trial
A 2-year trial in 160 subjects (females aged 18 to 40 years, males 18 to 50) with asthma receiving chlorofluorocarbon (CFC)-propelled fluticasone propionate inhalation aerosol 88 or 440 mcg twice daily demonstrated no statistically significant changes in BMD at any time point (24, 52, 76, and 104 weeks of double-blind treatment) as assessed by dual-energy x-ray absorptiometry at lumbar regions L1 through L4.
5.14 Effect on Growth
Orally inhaled corticosteroids may cause a reduction in growth velocity when administered to pediatric patients. Monitor the growth of pediatric patients receiving Fluticasone Propionate and Salmeterol HFA routinely (e.g., via stadiometry). To minimize the systemic effects of orally inhaled corticosteroids, including Fluticasone Propionate and Salmeterol HFA, titrate each patient’s dosage to the lowest dosage that effectively controls his/her symptoms [see Dosage and Administration (2), Use in Specific Populations (8.4)].
5.15 Glaucoma and Cataracts
Glaucoma, increased intraocular pressure, and cataracts have been reported in patients with asthma following the long-term administration of ICS, including fluticasone propionate, a component of Fluticasone Propionate and Salmeterol HFA. Consider referral to an ophthalmologist in patients who develop ocular symptoms or use Fluticasone Propionate and Salmeterol HFA long term [see Adverse Reactions (6)].
5.16 Eosinophilic Conditions and Churg-Strauss Syndrome
In rare cases, patients on inhaled fluticasone propionate, a component of Fluticasone Propionate and Salmeterol HFA, may present with systemic eosinophilic conditions. Some of these patients have clinical features of vasculitis consistent with Churg-Strauss syndrome, a condition that is often treated with systemic corticosteroid therapy. These events usually, but not always, have been associated with the reduction and/or withdrawal of oral corticosteroid therapy following the introduction of fluticasone propionate. Cases of serious eosinophilic conditions have also been reported with other ICS in this clinical setting. Physicians should be alert to eosinophilia, vasculitic rash, worsening pulmonary symptoms, cardiac complications, and/or neuropathy presenting in their patients. A causal relationship between fluticasone propionate and these underlying conditions has not been established.
5.17 Coexisting Conditions
Fluticasone Propionate and Salmeterol HFA, like all medicines containing sympathomimetic amines, should be used with caution in patients with convulsive disorders or thyrotoxicosis and in those who are unusually responsive to sympathomimetic amines. Large doses of the related beta2-adrenoceptor agonist albuterol, when administered intravenously, have been reported to aggravate preexisting diabetes mellitus and ketoacidosis.
Close5.18 Hypokalemia and Hyperglycemia
Beta-adrenergic agonist medicines may produce significant hypokalemia in some patients, possibly through intracellular shunting, which has the potential to produce adverse cardiovascular effects [see Clinical Pharmacology (12.2)]. The decrease in serum potassium is usually transient, not requiring supplementation. Clinically significant changes in blood glucose and/or serum potassium were seen infrequently during clinical trials with fluticasone propionate and salmeterol HFA at recommended doses.
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: • Serious asthma-related events – hospitalizations, intubations, death [see Warnings and ...
The following clinically significant adverse reactions are described elsewhere in the labeling:
- •
- Serious asthma-related events – hospitalizations, intubations, death [see Warnings and Precautions (5.1)]
- •
- Oropharyngeal candidiasis [see Warnings and Precautions (5.4)]
- •
- Pneumonia in patients with COPD [see Warnings and Precautions (5.5)]
- •
- Immunosuppression and risk of infections [see Warnings and Precautions (5.6)]
- •
- Hypercorticism and adrenal suppression [see Warnings and Precautions (5.8)]
- •
- Cardiovascular and central nervous system effects [see Warnings and Precautions (5.12)]
- •
- Reduction in bone mineral density [see Warnings and Precautions (5.13)]
- •
- Growth effects [see Warnings and Precautions (5.14)]
- •
- Glaucoma and cataracts [see Warnings and Precautions (5.15)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adult and Adolescent Subjects Aged 12 Years and Older
The incidence of adverse reactions associated with fluticasone propionate and salmeterol HFA in Table 2 is based upon two 12-week, placebo-controlled, U.S. clinical trials (Trials 1 and 3) and 1 active-controlled 12-week U.S. clinical trial (Trial 2). A total of 1,008 adult and adolescent subjects with asthma (556 females and 452 males) previously treated with albuterol alone, salmeterol, or ICS were treated twice daily with 2 inhalations of fluticasone propionate and salmeterol HFA 45 mcg/21 mcg or fluticasone propionate and salmeterol HFA 115 mcg/21 mcg, fluticasone propionate CFC inhalation aerosol (44- or 110-mcg doses), salmeterol CFC inhalation aerosol 21 mcg, or placebo HFA inhalation aerosol. The average duration of exposure was 71 to 81 days in the active treatment groups compared with 51 days in the placebo group.
Table 2. Adverse Reactions with Fluticasone Propionate and Salmeterol HFA with ≥3% Incidence in Adult and Adolescent Subjects with Asthma - Adverse Event
Fluticasone Propionate and Salmeterol HFA
Fluticasone Propionate CFC Inhalation Aerosol
Salmeterol CFC Inhalation Aerosol
Placebo HFA Inhalation Aerosol
- 45 mcg/21 mcg
- (n = 187)
- %
- 115 mcg/21 mcg
- (n = 94)
- %
- 44 mcg
- (n = 186)
- %
- 110 mcg
- (n = 91)
- %
- 21 mcg
- (n = 274)
- %
- (n = 176)
- %
Ear, nose, and throat
Upper respiratory tract infection
16
24
13
15
17
13
Throat irritation
9
7
12
13
9
7
Upper respiratory inflammation
4
4
3
7
5
3
Hoarseness/dysphonia
3
1
2
0
1
0
Lower respiratory
Viral respiratory infection
3
5
4
5
3
4
Neurology
Headache
21
15
24
16
20
11
Dizziness
4
1
1
0
<1
0
Gastrointestinal
Nausea and vomiting
5
3
4
2
2
3
Viral gastrointestinal infection
4
2
2
0
1
2
Gastrointestinal signs and symptoms
3
2
2
1
1
1
Musculoskeletal
Musculoskeletal pain
5
7
8
2
4
4
Muscle pain
4
1
1
1
3
<1
The incidence of common adverse reactions reported in Trial 4, a 12-week non-U.S. clinical trial in 509 subjects previously treated with ICS who were treated twice daily with 2 inhalations of fluticasone propionate and salmeterol HFA 230 mcg/21 mcg, fluticasone propionate CFC inhalation aerosol 220 mcg, or 1 inhalation of ADVAIR DISKUS 500 mcg/50 mcg was similar to the incidences reported in Table 2.
Additional Adverse Reactions
Other adverse reactions not previously listed, whether considered drug-related or not by the investigators, that occurred in the groups receiving fluticasone propionate and salmeterol HFA with an incidence of 1% to 3% and that occurred at a greater incidence than with placebo include the following: tachycardia, arrhythmias, myocardial infarction, postoperative complications, wounds and lacerations, soft tissue injuries, ear signs and symptoms, rhinorrhea/postnasal drip, epistaxis, nasal congestion/blockage, laryngitis, unspecified oropharyngeal plaques, dryness of nose, weight gain, allergic eye disorders, eye edema and swelling, gastrointestinal discomfort and pain, dental discomfort and pain, candidiasis mouth/throat, hyposalivation, gastrointestinal infections, disorders of hard tissue of teeth, abdominal discomfort and pain, oral abnormalities, arthralgia and articular rheumatism, muscle cramps and spasms, musculoskeletal inflammation, bone and skeletal pain, muscle injuries, sleep disorders, migraines, allergies and allergic reactions, viral infections, bacterial infections, candidiasis unspecified site, congestion, inflammation, bacterial reproductive infections, lower respiratory signs and symptoms, lower respiratory infections, lower respiratory hemorrhage, eczema, dermatitis and dermatosis, urinary infections.
Laboratory Test Abnormalities
In Trial 3, there were more reports of hyperglycemia among adults and adolescents receiving fluticasone propionate and salmeterol HFA, but this was not seen in Trials 1 and 2.
Close6.2 Postmarketing Experience
In addition to adverse reactions reported from clinical trials, the following adverse reactions have been identified during postapproval use of any formulation of fluticasone propionate and salmeterol, fluticasone propionate, and/or salmeterol regardless of indication. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These events have been chosen for inclusion due to either their seriousness, frequency of reporting, or causal connection to fluticasone propionate and salmeterol, fluticasone propionate, and/or salmeterol or a combination of these factors.
Cardiovascular
Arrhythmias (including atrial fibrillation, extrasystoles, supraventricular tachycardia), hypertension, ventricular tachycardia.
Ear, Nose, and Throat
Aphonia, earache, facial and oropharyngeal edema, paranasal sinus pain, rhinitis, throat soreness, tonsillitis.
Endocrine and Metabolic
Cushing’s syndrome, Cushingoid features, growth velocity reduction in children/adolescents, hypercorticism, osteoporosis.
Eye
Cataracts, glaucoma.
Gastrointestinal
Dyspepsia, xerostomia.
Hepatobiliary Tract and Pancreas
Abnormal liver function tests.
Immune System
Immediate and delayed hypersensitivity reactions, including rash and rare events of angioedema, bronchospasm, and anaphylaxis.
Infections and Infestations
Esophageal candidiasis.
Musculoskeletal
Back pain, myositis.
Neurology
Paresthesia, restlessness.
Non-Site Specific
Fever, pallor.
Psychiatry
Agitation, aggression, anxiety, depression. Behavioral changes, including hyperactivity and irritability, have been reported very rarely and primarily in children.
Respiratory
Asthma; asthma exacerbation; chest congestion; chest tightness; cough; dyspnea; immediate bronchospasm; influenza; paradoxical bronchospasm; tracheitis; wheezing; pneumonia; reports of upper respiratory symptoms of laryngeal spasm, irritation, or swelling such as stridor or choking.
Skin
Contact dermatitis, contusions, ecchymoses, photodermatitis, pruritus.
Urogenital
Dysmenorrhea, irregular menstrual cycle, pelvic inflammatory disease, vaginal candidiasis, vaginitis, vulvovaginitis.
-
7 DRUG INTERACTIONSFluticasone propionate and salmeterol HFA has been used concomitantly with other drugs, including short-acting beta2-agonists, methylxanthines, and nasal corticosteroids, commonly used in patients ...
Fluticasone propionate and salmeterol HFA has been used concomitantly with other drugs, including short-acting beta2-agonists, methylxanthines, and nasal corticosteroids, commonly used in patients with asthma without adverse drug reactions [see Clinical Pharmacology (12.2)]. No formal drug interaction trials have been performed with fluticasone propionate and salmeterol HFA.
7.1 Inhibitors of Cytochrome P450 3A4
Fluticasone propionate and salmeterol, the individual components of Fluticasone Propionate and Salmeterol HFA, are substrates of CYP3A4. The use of strong CYP3A4 inhibitors (e.g., ritonavir, atazanavir, clarithromycin, indinavir, itraconazole, nefazodone, nelfinavir, saquinavir, ketoconazole, telithromycin) with Fluticasone Propionate and Salmeterol HFA is not recommended because increased systemic corticosteroid and increased cardiovascular adverse effects may occur.
Ritonavir
Fluticasone Propionate: A drug interaction trial with fluticasone propionate aqueous nasal spray in healthy subjects has shown that ritonavir (a strong CYP3A4 inhibitor) can significantly increase plasma fluticasone propionate exposure, resulting in significantly reduced serum cortisol concentrations [see Clinical Pharmacology (12.3)]. During postmarketing use, there have been reports of clinically significant drug interactions in patients receiving fluticasone propionate and ritonavir, resulting in systemic corticosteroid effects including Cushing’s syndrome and adrenal suppression.
Ketoconazole
Fluticasone Propionate: Coadministration of orally inhaled fluticasone propionate (1,000 mcg) and ketoconazole (200 mg once daily) resulted in a 1.9-fold increase in plasma fluticasone propionate exposure and a 45% decrease in plasma cortisol area under the curve (AUC), but had no effect on urinary excretion of cortisol.
Salmeterol: In a drug interaction trial in 20 healthy subjects, coadministration of inhaled salmeterol (50 mcg twice daily) and oral ketoconazole (400 mg once daily) for 7 days resulted in greater systemic exposure to salmeterol (AUC increased 16-fold and Cmax increased 1.4-fold). Three (3) subjects were withdrawn due to beta2-agonist side effects (2 with prolonged QTc and 1 with palpitations and sinus tachycardia). Although there was no statistical effect on the mean QTc, coadministration of salmeterol and ketoconazole was associated with more frequent increases in QTc duration compared with salmeterol and placebo administration.
7.2 Monoamine Oxidase Inhibitors and Tricyclic Antidepressants
Fluticasone Propionate and Salmeterol HFA should be administered with extreme caution to patients being treated with monoamine oxidase inhibitors or tricyclic antidepressants, or within 2 weeks of discontinuation of such agents, because the action of salmeterol, a component of Fluticasone Propionate and Salmeterol HFA, on the vascular system may be potentiated by these agents.
7.3 Beta-adrenergic Receptor Blocking Agents
Beta-blockers not only block the pulmonary effect of beta-agonists, such as salmeterol, a component of Fluticasone Propionate and Salmeterol HFA, but may also produce severe bronchospasm in patients with asthma. Therefore, patients with asthma should not normally be treated with beta-blockers. However, under certain circumstances, there may be no acceptable alternatives to the use of beta-adrenergic blocking agents for these patients; cardioselective beta-blockers could be considered, although they should be administered with caution.
Close7.4 Non–Potassium-Sparing Diuretics
The ECG changes and/or hypokalemia that may result from the administration of non–potassium-sparing diuretics (such as loop or thiazide diuretics) can be acutely worsened by beta-agonists, such as salmeterol, a component of Fluticasone Propionate and Salmeterol HFA, especially when the recommended dose of the beta-agonist is exceeded. Although the clinical significance of these effects is not known, caution is advised in the coadministration of Fluticasone Propionate and Salmeterol HFA with non–potassium-sparing diuretics.
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are insufficient data on the use of fluticasone propionate and salmeterol HFA or individual monoproducts, fluticasone propionate and salmeterol xinafoate, in ...
8.1 Pregnancy
Risk Summary
There are insufficient data on the use of fluticasone propionate and salmeterol HFA or individual monoproducts, fluticasone propionate and salmeterol xinafoate, in pregnant women. There are clinical considerations with the use of fluticasone propionate and salmeterol HFA in pregnant women. (See Clinical Considerations.) In animals, teratogenicity characteristic of corticosteroids, decreased fetal body weight and/or skeletal variations, in rats, mice, and rabbits, was observed with subcutaneously administered maternal toxic doses of fluticasone propionate less than the maximum recommended human daily inhaled dose (MRHDID) on a mcg/m2 basis. (See Data.) However, fluticasone propionate administered via inhalation to rats decreased fetal body weight but did not induce teratogenicity at a maternal toxic dose less than the MRHDID on a mcg/m2 basis. (See Data.) Experience with oral corticosteroids suggests that rodents are more prone to teratogenic effects from corticosteroids than humans. Oral administration of salmeterol to pregnant rabbits caused teratogenicity characteristic of beta-adrenoceptor stimulation at maternal doses approximately 25 times the MRHDID on an AUC basis. These adverse effects generally occurred at large multiples of the MRHDID when salmeterol was administered by the oral route to achieve high systemic exposures. No such effects occurred at an oral salmeterol dose approximately 10 times the MRHDID. (See Data.)
The estimated risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Disease-Associated Maternal and/or Embryofetal Risk: In women with poorly or moderately controlled asthma, there is an increased risk of several perinatal outcomes such as pre-eclampsia in the mother and prematurity, low birth weight, and small for gestational age in the neonate. Pregnant women should be closely monitored and medication adjusted as necessary to maintain optimal control of asthma.
Labor and Delivery: There are no human studies evaluating the effects of fluticasone propionate and salmeterol HFA during labor and delivery. Because of the potential for beta-agonist interference with uterine contractility, use of Fluticasone Propionate and Salmeterol HFA during labor should be restricted to those patients in whom the benefits clearly outweigh the risks.
Data
Human Data: Fluticasone Propionate: Following inhaled administration, fluticasone propionate was detected in the neonatal cord blood after delivery.
Animal Data: Fluticasone Propionate and Salmeterol: In an embryofetal development study with pregnant rats that received the combination of subcutaneous administration of fluticasone propionate and oral administration of salmeterol at doses of 0/1,000; 30/0; 10/100; 30/1,000; and 100/10,000 mcg/kg/day (as fluticasone propionate/salmeterol) during the period of organogenesis, findings were generally consistent with the individual monoproducts and there was no exacerbation of expected fetal effects. Omphalocele, increased embryofetal deaths, decreased body weight, and skeletal variations were observed in rat fetuses in the presence of maternal toxicity when combining fluticasone propionate at a dose approximately equivalent to the MRHDID (on a mcg/m2 basis at a maternal subcutaneous dose of 100 mcg/kg/day) and salmeterol at a dose approximately 1,200 times the MRHDID (on a mcg/m2 basis at a maternal oral dose of 10,000 mcg/kg/day). The rat no observed adverse effect level (NOAEL) was observed when combining fluticasone propionate at a dose less than the MRHDID (on a mcg/m2 basis at a maternal subcutaneous dose of 30 mcg/kg/day) and salmeterol at a dose approximately 120 times the MRHDID (on a mcg/m2 basis at a maternal oral dose of 1,000 mcg/kg/day).
In an embryofetal development study with pregnant mice that received the combination of subcutaneous administration of fluticasone propionate and oral administration of salmeterol at doses of 0/1,400; 40/0; 10/200; 40/1,400; or 150/10,000 mcg/kg/day (as fluticasone propionate/salmeterol) during the period of organogenesis, findings were generally consistent with the individual monoproducts and there was no exacerbation of expected fetal effects. Cleft palate, fetal death, increased implantation loss, and delayed ossification were observed in mouse fetuses when combining fluticasone propionate at a dose approximately equivalent to the MRHDID (on a mcg/m2 basis at a maternal subcutaneous dose of 150 mcg/kg/day) and salmeterol at a dose approximately 580 times the MRHDID (on a mcg/m2 basis at a maternal oral dose of 10,000 mcg/kg/day). No developmental toxicity was observed at combination doses of fluticasone propionate up to approximately 0.2 times the MRHDID (on a mcg/m2 basis at a maternal subcutaneous dose of 40 mcg/kg) and doses of salmeterol up to approximately 80 times the MRHDID (on a mcg/m2 basis at a maternal oral dose of 1,400 mcg/kg).
Fluticasone Propionate: In embryofetal development studies with pregnant rats and mice dosed by the subcutaneous route throughout the period of organogenesis, fluticasone propionate was teratogenic in both species. Omphalocele, decreased body weight, and skeletal variations were observed in rat fetuses, in the presence of maternal toxicity, at a dose approximately equivalent to the MRHDID (on a mcg/m2 basis with a maternal subcutaneous dose of 100 mcg/kg/day). The rat NOAEL was observed at approximately 0.3 times the MRHDID (on a mcg/m2 basis with a maternal subcutaneous dose of 30 mcg/kg/day). Cleft palate and fetal skeletal variations were observed in mouse fetuses at a dose approximately 0.2 times the MRHDID (on a mcg/m2 basis with a maternal subcutaneous dose of 45 mcg/kg/day). The mouse NOAEL was observed with a dose approximately 0.08 times the MRHDID (on a mcg/m2 basis with a maternal subcutaneous dose of 15 mcg/kg/day).
In an embryofetal development study with pregnant rats dosed by the inhalation route throughout the period of organogenesis, fluticasone propionate produced decreased fetal body weights and skeletal variations, in the presence of maternal toxicity, at a dose approximately 0.3 times the MRHDID (on a mcg/m2 basis with a maternal inhalation dose of 25.7 mcg/kg/day); however, there was no evidence of teratogenicity. The NOAEL was observed with a dose approximately 0.05 times the MRHDID (on a mcg/m2 basis with a maternal inhalation dose of 5.5 mcg/kg/day).
In an embryofetal development study in pregnant rabbits that were dosed by the subcutaneous route throughout organogenesis, fluticasone propionate produced reductions of fetal body weights, in the presence of maternal toxicity, at doses less than the MRHDID (on a mcg/m2 basis with a maternal subcutaneous dose of 0.57 mcg/kg/day). Teratogenicity was evident based upon a finding of cleft palate for 1 fetus at a dose approximately 0.09 times the MRHDID (on a mcg/m2 basis with a maternal subcutaneous dose of 4 mcg/kg/day). The NOAEL was observed in rabbit fetuses with a dose approximately 0.002 times the MRHDID (on a mcg/m2 basis with a maternal subcutaneous dose of 0.08 mcg/kg/day).
Fluticasone propionate crossed the placenta following subcutaneous administration to mice and rats and oral administration to rabbits.
In a pre- and post-natal development study in pregnant rats dosed by the subcutaneous route from late gestation through delivery and lactation (Gestation Day 17 to Postpartum Day 22), fluticasone propionate was not associated with decreases in pup body weight and had no effects on developmental landmarks, learning, memory, reflexes, or fertility at doses up to 0.5 times the MRHDID (on a mcg/m2 basis with maternal subcutaneous doses up to 50 mcg/kg/day).
Salmeterol: In 3 embryofetal development studies, pregnant rabbits received oral administration of salmeterol at doses ranging from 100 to 10,000 mcg/kg/day during the period of organogenesis. In pregnant Dutch rabbits administered salmeterol doses approximately 25 times the MRHDID (on an AUC basis at maternal oral doses of 1,000 mcg/kg/day and higher), fetal toxic effects were observed characteristically resulting from beta-adrenoceptor stimulation. These included precocious eyelid openings, cleft palate, sternebral fusion, limb and paw flexures, and delayed ossification of the frontal cranial bones. No such effects occurred at a salmeterol dose approximately 10 times the MRHDID (on an AUC basis at a maternal oral dose of 600 mcg/kg/day). New Zealand White rabbits were less sensitive since only delayed ossification of the frontal cranial bones was seen at a salmeterol dose approximately 2,300 times the MRHDID (on a mcg/m2 basis at a maternal oral dose of 10,000 mcg/kg/day).
In 2 embryofetal development studies, pregnant rats received salmeterol by oral administration at doses ranging from 100 to 10,000 mcg/kg/day during the period of organogenesis. Salmeterol produced no maternal toxicity or embryofetal effects at doses up to approximately 1,200 times the MRHDID (on a mcg/m2 basis at maternal oral doses up to 10,000 mcg/kg/day).
In a peri- and post-natal development study in pregnant rats dosed by the oral route from late gestation through delivery and lactation, salmeterol at a dose approximately 1,200 times the MRHDID (on a mcg/m2 basis with a maternal oral dose of 10,000 mcg/kg/day) was fetotoxic and decreased the fertility of survivors.
Salmeterol xinafoate crossed the placenta following oral administration to mice and rats.
8.2 Lactation
Risk Summary
There are no available data on the presence of fluticasone propionate or salmeterol in human milk, the effects on the breastfed child, or the effects on milk production. Other corticosteroids have been detected in human milk. However, fluticasone propionate and salmeterol concentrations in plasma after inhaled therapeutic doses are low and therefore concentrations in human breast milk are likely to be correspondingly low [see Clinical Pharmacology (12.3)]. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Fluticasone Propionate and Salmeterol HFA and any potential adverse effects on the breastfed child from Fluticasone Propionate and Salmeterol HFA or from the underlying maternal condition.
Data
Animal Data: Subcutaneous administration of tritiated fluticasone propionate at a dose of 10 mcg/kg/day to lactating rats resulted in measurable levels in milk. Oral administration of salmeterol at a dose of 10,000 mcg/kg/day to lactating rats resulted in measurable levels in milk.
8.4 Pediatric Use
The safety and effectiveness of fluticasone propionate and salmeterol HFA have been established in pediatric patients aged 12 years and older. Use of fluticasone propionate and salmeterol HFA in this age group is supported by evidence from adequate and well-controlled studies in adults with additional data from thirty-eight (38) subjects aged 12 to 17 years were treated with fluticasone propionate and salmeterol HFA in U.S. pivotal clinical trials. Subjects in this age group demonstrated efficacy results similar to those observed in subjects aged 18 years and older. There were no obvious differences in the type or frequency of adverse events reported in this age group compared with subjects aged 18 years and older.
In a 12-week trial, the safety of fluticasone propionate and salmeterol HFA 45 mcg/21 mcg given as 2 inhalations twice daily was compared with that of fluticasone propionate 44 mcg HFA (FLOVENT HFA [fluticasone propionate inhalation aerosol]) 2 inhalations twice daily in 350 subjects aged 4 to 11 years with persistent asthma currently being treated with ICS. No new safety concerns were observed in children aged 4 to 11 years treated for 12 weeks with fluticasone propionate and salmeterol HFA 45 mcg/21 mcg compared with adults and adolescents aged 12 years and older. Common adverse reactions (≥3%) seen in children aged 4 to 11 years treated with fluticasone propionate and salmeterol HFA 45 mcg/21 mcg but not reported in the adult and adolescent clinical trials of fluticasone propionate and salmeterol HFA include: pyrexia, cough, pharyngolaryngeal pain, rhinitis, and sinusitis [see Adverse Reactions (6.1)]. This trial was not designed to assess the effect of salmeterol, a component of Fluticasone Propionate and Salmeterol HFA, on asthma hospitalizations and death in subjects aged 4 to 11 years.
The pharmacokinetics and pharmacodynamic effect on serum cortisol of 21 days of treatment with fluticasone propionate and salmeterol HFA 45 mcg/21 mcg (2 inhalations twice daily with or without a spacer) or ADVAIR DISKUS 100 mcg/50 mcg (1 inhalation twice daily) was evaluated in a trial of 31 children aged 4 to 11 years with mild asthma. Systemic exposure to salmeterol xinafoate was similar for fluticasone propionate and salmeterol HFA, fluticasone propionate and salmeterol HFA delivered with a spacer, and ADVAIR DISKUS while the systemic exposure to fluticasone propionate was lower with fluticasone propionate and salmeterol HFA compared with that of fluticasone propionate and salmeterol HFA delivered with a spacer or ADVAIR DISKUS. There were reductions in serum cortisol from baseline in all treatment groups (14%, 22%, and 13% for fluticasone propionate and salmeterol HFA, fluticasone propionate and salmeterol HFA delivered with a spacer, and ADVAIR DISKUS, respectively) [see Clinical Pharmacology (12.2, 12.3)].
The safety and effectiveness of fluticasone propionate and salmeterol HFA in pediatric patients younger than 12 years have not been established.
Effects on Growth
ICS, including fluticasone propionate, a component of Fluticasone Propionate and Salmeterol HFA, may cause a reduction in growth velocity in children and adolescents [see Warnings and Precautions (5.14)]. The growth of pediatric patients receiving orally inhaled corticosteroids, including fluticasone propionate and salmeterol HFA, should be monitored.
A 52-week placebo-controlled trial to assess the potential growth effects of fluticasone propionate inhalation powder (FLOVENT ROTADISK) at 50 and 100 mcg twice daily was conducted in the U.S. in 325 prepubescent children (244 males and 81 females) aged 4 to 11 years. The mean growth velocities at 52 weeks observed in the intent-to-treat population were 6.32 cm/year in the placebo group (n = 76), 6.07 cm/year in the 50-mcg group (n = 98), and 5.66 cm/year in the 100-mcg group (n = 89). An imbalance in the proportion of children entering puberty between groups and a higher dropout rate in the placebo group due to poorly controlled asthma may be confounding factors in interpreting these data. A separate subset analysis of children who remained prepubertal during the trial revealed growth rates at 52 weeks of 6.10 cm/year in the placebo group (n = 57), 5.91 cm/year in the 50-mcg group (n = 74), and 5.67 cm/year in the 100-mcg group (n = 79). In children aged 8.5 years, the mean age of children in this trial, the range for expected growth velocity is: boys – 3rd percentile = 3.8 cm/year, 50th percentile = 5.4 cm/year, and 97th percentile = 7.0 cm/year; girls – 3rd percentile = 4.2 cm/year, 50th percentile = 5.7 cm/year, and 97th percentile = 7.3 cm/year. The clinical relevance of these growth data is not certain.
If a child or adolescent on any corticosteroid appears to have growth suppression, the possibility that he/she is particularly sensitive to this effect of corticosteroids should be considered. The potential growth effects of prolonged treatment should be weighed against the clinical benefits obtained. To minimize the systemic effects of orally inhaled corticosteroids, including fluticasone propionate and salmeterol HFA, each patient should be titrated to the lowest strength that effectively controls his/her asthma [see Dosage and Administration (2)].
8.5 Geriatric Use
Clinical trials of fluticasone propionate and salmeterol HFA did not include sufficient numbers of subjects aged 65 years and older to determine whether older subjects respond differently than younger subjects. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. In addition, as with other products containing beta2-agonists, special caution should be observed when using Fluticasone Propionate and Salmeterol HFA in geriatric patients who have concomitant cardiovascular disease that could be adversely affected by beta2-agonists.
8.6 Hepatic Impairment
Formal pharmacokinetic studies using fluticasone propionate and salmeterol HFA have not been conducted in patients with hepatic impairment. However, since both fluticasone propionate and salmeterol are predominantly cleared by hepatic metabolism, impairment of liver function may lead to accumulation of fluticasone propionate and salmeterol in plasma. Therefore, patients with hepatic disease should be closely monitored.
Close8.7 Renal Impairment
Formal pharmacokinetic studies using fluticasone propionate and salmeterol HFA have not been conducted in patients with renal impairment.
-
10 OVERDOSAGENo human overdosage data has been reported for fluticasone propionate and salmeterol HFA. Fluticasone Propionate and Salmeterol HFA contains both fluticasone propionate and salmeterol; therefore ...
No human overdosage data has been reported for fluticasone propionate and salmeterol HFA.
Fluticasone Propionate and Salmeterol HFA contains both fluticasone propionate and salmeterol; therefore, the risks associated with overdosage for the individual components described below apply to Fluticasone Propionate and Salmeterol HFA. Treatment of overdosage consists of discontinuation of Fluticasone Propionate and Salmeterol HFA together with institution of appropriate symptomatic and/or supportive therapy. The judicious use of a cardioselective beta-receptor blocker may be considered, bearing in mind that such medication can produce bronchospasm. Cardiac monitoring is recommended in cases of overdosage.
Fluticasone Propionate
Chronic overdosage of fluticasone propionate may result in signs/symptoms of hypercorticism [see Warnings and Precautions (5.8)].
Salmeterol
The expected signs and symptoms with overdosage of salmeterol are those of excessive beta‑adrenergic stimulation and/or occurrence or exaggeration of any of the signs and symptoms of beta-adrenergic stimulation (e.g., seizures, angina, hypertension or hypotension, tachycardia with rates up to 200 beats/min, arrhythmias, nervousness, headache, tremor, muscle cramps, dry mouth, palpitation, nausea, dizziness, fatigue, malaise, insomnia, hyperglycemia, hypokalemia, metabolic acidosis). Overdosage with salmeterol can lead to clinically significant prolongation of the QTc interval, which can produce ventricular arrhythmias.
As with all inhaled sympathomimetic medicines, cardiac arrest and even death may be associated with an overdose of salmeterol.
Close -
11 DESCRIPTIONFluticasone Propionate and Salmeterol HFA 45 mcg/21 mcg inhalation aerosol, Fluticasone Propionate and Salmeterol HFA inhalation aerosol 115 mcg/21 mcg, and Fluticasone Propionate and Salmeterol ...
Fluticasone Propionate and Salmeterol HFA 45 mcg/21 mcg inhalation aerosol, Fluticasone Propionate and Salmeterol HFA inhalation aerosol 115 mcg/21 mcg, and Fluticasone Propionate and Salmeterol HFA inhalation aerosol 230 mcg/21 mcg are combinations of fluticasone propionate and salmeterol xinafoate.
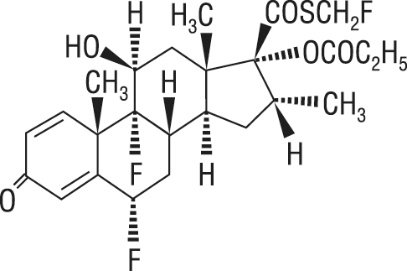
One active component of Fluticasone Propionate and Salmeterol HFA is fluticasone propionate, a corticosteroid having the chemical name S-(fluoromethyl) 6α,9-difluoro-11β,17-dihydroxy-16α-methyl-3-oxoandrosta-1,4-diene-17β-carbothioate, 17-propionate and the following chemical structure:
Fluticasone propionate is a white powder with a molecular weight of 500.6, and the empirical formula is C25H31F3O5S. It is practically insoluble in water, freely soluble in dimethyl sulfoxide and dimethylformamide, and slightly soluble in methanol and 95% ethanol.
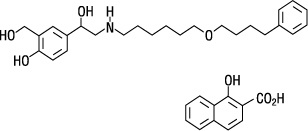
The other active component of Fluticasone Propionate and Salmeterol HFA is salmeterol xinafoate, a beta2-adrenergic bronchodilator. Salmeterol xinafoate is the racemic form of the 1-hydroxy-2-naphthoic acid salt of salmeterol. It has the chemical name 4-hydroxy-α1-[[[6-(4-phenylbutoxy)hexyl]amino]methyl]-1,3-benzenedimethanol, 1-hydroxy-2-naphthalenecarboxylate and the following chemical structure:
Salmeterol xinafoate is a white powder with a molecular weight of 603.8, and the empirical formula is C25H37NO4•C11H8O3. It is freely soluble in methanol; slightly soluble in ethanol, chloroform, and isopropanol; and sparingly soluble in water.
Fluticasone Propionate and Salmeterol HFA is a purple plastic inhaler with a light purple cap containing a pressurized metered-dose aerosol canister fitted with a counter. Each canister contains a microcrystalline suspension of micronized fluticasone propionate and micronized salmeterol xinafoate in propellant HFA-134a (1,1,1,2-tetrafluoroethane). It contains no other excipients.
After priming, each actuation of the inhaler delivers 50, 125, or 250 mcg of fluticasone propionate and 25 mcg of salmeterol in 75 mg of suspension from the valve. Each actuation delivers 45, 115, or 230 mcg of fluticasone propionate and 21 mcg of salmeterol from the actuator. Twenty-one micrograms (21 mcg) of salmeterol base is equivalent to 30.45 mcg of salmeterol xinafoate. The actual amount of drug delivered to the lung will depend on patient factors, such as the coordination between the actuation of the inhaler and inspiration through the delivery system.
Prime Fluticasone Propionate and Salmeterol HFA before using for the first time by releasing 4 sprays into the air away from the face, shaking well for 5 seconds before each spray. In cases where the inhaler has not been used for more than 4 weeks or when it has been dropped, prime the inhaler again by releasing 2 sprays into the air away from the face, shaking well for 5 seconds before each spray. Avoid spraying in eyes.
Close -
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Fluticasone Propionate and Salmeterol HFA - Fluticasone Propionate and Salmeterol HFA contains both fluticasone propionate and salmeterol. The mechanisms of action ...
12.1 Mechanism of Action
Fluticasone Propionate and Salmeterol HFA
Fluticasone Propionate and Salmeterol HFA contains both fluticasone propionate and salmeterol. The mechanisms of action described below for the individual components apply to Fluticasone Propionate and Salmeterol HFA. These drugs represent 2 different classes of medications (a synthetic corticosteroid and a LABA) that have different effects on clinical, physiologic, and inflammatory indices of asthma.
Fluticasone Propionate
Fluticasone propionate is a synthetic trifluorinated corticosteroid with anti-inflammatory activity. Fluticasone propionate has been shown in vitro to exhibit a binding affinity for the human glucocorticoid receptor that is 18 times that of dexamethasone, almost twice that of beclomethasone-17-monopropionate (BMP), the active metabolite of beclomethasone dipropionate, and over 3 times that of budesonide. Data from the McKenzie vasoconstrictor assay in man are consistent with these results. The clinical significance of these findings is unknown.
Inflammation is an important component in the pathogenesis of asthma. Corticosteroids have been shown to have a wide range of actions on multiple cell types (e.g., mast cells, eosinophils, neutrophils, macrophages, lymphocytes) and mediators (e.g., histamine, eicosanoids, leukotrienes, cytokines) involved in inflammation. These anti-inflammatory actions of corticosteroids contribute to their efficacy in asthma.
Salmeterol Xinafoate
Salmeterol is a selective LABA. In vitro studies show salmeterol to be at least 50 times more selective for beta2‑adrenoceptors than albuterol. Although beta2-adrenoceptors are the predominant adrenergic receptors in bronchial smooth muscle and beta1-adrenoceptors are the predominant receptors in the heart, there are also beta2-adrenoceptors in the human heart comprising 10% to 50% of the total beta-adrenoceptors. The precise function of these receptors has not been established, but their presence raises the possibility that even selective beta2-agonists may have cardiac effects.
The pharmacologic effects of beta2-adrenoceptor agonist drugs, including salmeterol, are at least in part attributable to stimulation of intracellular adenyl cyclase, the enzyme that catalyzes the conversion of adenosine triphosphate (ATP) to cyclic-3′,5′-adenosine monophosphate (cyclic AMP). Increased cyclic AMP levels cause relaxation of bronchial smooth muscle and inhibition of release of mediators of immediate hypersensitivity from cells, especially from mast cells.
In vitro tests show that salmeterol is a potent and long-lasting inhibitor of the release of mast cell mediators, such as histamine, leukotrienes, and prostaglandin D2, from human lung. Salmeterol inhibits histamine-induced plasma protein extravasation and inhibits platelet-activating factor–induced eosinophil accumulation in the lungs of guinea pigs when administered by the inhaled route. In humans, single doses of salmeterol administered via inhalation aerosol attenuate allergen-induced bronchial hyper-responsiveness.
12.2 Pharmacodynamics
Fluticasone Propionate and Salmeterol HFA
Healthy Subjects: Cardiovascular Effects: Since systemic pharmacodynamic effects of salmeterol are not normally seen at the therapeutic dose, higher doses were used to produce measurable effects. Four (4) placebo-controlled crossover trials were conducted with healthy subjects: (1) a cumulative-dose trial using 42 to 336 mcg of salmeterol CFC inhalation aerosol given alone or as fluticasone propionate and salmeterol HFA 115 mcg/21 mcg, (2) a single-dose trial using 4 inhalations of fluticasone propionate and salmeterol HFA 230 mcg/21 mcg, salmeterol CFC inhalation aerosol 21 mcg, or fluticasone propionate CFC inhalation aerosol 220 mcg, (3) a single-dose trial using 8 inhalations of fluticasone propionate and salmeterol HFA 45 mcg/21 mcg, fluticasone propionate and salmeterol HFA 115 mcg/21 mcg, or fluticasone propionate and salmeterol HFA 230 mcg/21 mcg, and (4) a single-dose trial using 4 inhalations of fluticasone propionate and salmeterol HFA 230 mcg/21 mcg; 2 inhalations of ADVAIR DISKUS 500 mcg/50 mcg; 4 inhalations of fluticasone propionate CFC inhalation aerosol 220 mcg; or 1,010 mcg of fluticasone propionate given intravenously. In these trials pulse rate, blood pressure, QTc interval, glucose, and/or potassium were measured. Comparable or lower effects were observed for fluticasone propionate and salmeterol HFA compared with ADVAIR DISKUS or salmeterol alone. The effect of salmeterol on pulse rate and potassium was not altered by the presence of different amounts of fluticasone propionate in fluticasone propionate and salmeterol HFA.
Hypothalamic-Pituitary-Adrenal Axis Effects: The potential effect of salmeterol on the effects of fluticasone propionate on the HPA axis was also evaluated in 3 of these trials. Compared with fluticasone propionate CFC inhalation aerosol, fluticasone propionate and salmeterol HFA had less effect on 24-hour urinary cortisol excretion and less or comparable effect on 24-hour serum cortisol. In these crossover trials in healthy subjects, fluticasone propionate and salmeterol HFA and ADVAIR DISKUS had similar effects on urinary and serum cortisol.
Subjects with Asthma: Cardiovascular Effects: In clinical trials with fluticasone propionate and salmeterol HFA in adult and adolescent subjects aged 12 years and older with asthma, systemic pharmacodynamic effects of salmeterol (pulse rate, blood pressure, QTc interval, potassium, and glucose) were similar to or slightly lower in patients treated with fluticasone propionate and salmeterol HFA compared with patients treated with salmeterol CFC inhalation aerosol 21 mcg. In 61 adult and adolescent subjects with asthma given fluticasone propionate and salmeterol HFA (45 mcg/21 mcg or 115 mcg/21 mcg), continuous 24-hour electrocardiographic monitoring was performed after the first dose and after 12 weeks of twice-daily therapy, and no clinically significant dysrhythmias were noted.
The effect of 21 days of treatment with fluticasone propionate and salmeterol HFA 45 mcg/21 mcg (2 inhalations twice daily with or without a spacer) or ADVAIR DISKUS 100 mcg/50 mcg (1 inhalation twice daily) was evaluated in 31 children aged 4 to 11 years with mild asthma. There were no notable changes from baseline for QTc, heart rate, or systolic and diastolic blood pressure.
Hypothalamic-Pituitary-Adrenal Axis Effects: A 4-way crossover trial in 13 subjects with asthma compared pharmacodynamics at steady state following 4 weeks of twice-daily treatment with 2 inhalations of fluticasone propionate and salmeterol HFA 115 mcg/21 mcg, 1 inhalation of ADVAIR DISKUS 250 mcg/50 mcg, 2 inhalations of fluticasone propionate HFA inhalation aerosol 110 mcg, and placebo. No significant differences in serum cortisol AUC were observed between active treatments and placebo. Mean 12-hour serum cortisol AUC ratios comparing active treatment with placebo ranged from 0.9 to 1.2. No statistically or clinically significant increases in heart rate or QTc interval were observed for any active treatment compared with placebo.
In a 12-week trial in adult and adolescent subjects with asthma, fluticasone propionate and salmeterol HFA 115 mcg/21 mcg was compared with the individual components, fluticasone propionate CFC inhalation aerosol 110 mcg and salmeterol CFC inhalation aerosol 21 mcg, and placebo [see Clinical Studies (14.1)]. All treatments were administered as 2 inhalations twice daily. After 12 weeks of treatment with these therapeutic doses, the geometric mean ratio of urinary cortisol excretion compared with baseline was 0.9 for fluticasone propionate and salmeterol HFA and fluticasone propionate and 1.0 for placebo and salmeterol. In addition, the ability to increase cortisol production in response to stress, as assessed by 30-minute cosyntropin stimulation in 23 to 32 subjects per treatment group, remained intact for the majority of subjects and was similar across treatments. Three (3) subjects who received fluticasone propionate and salmeterol HFA 115 mcg/21 mcg had an abnormal response (peak serum cortisol <18 mcg/dL) after dosing, compared with 1 subject who received placebo, 2 subjects who received fluticasone propionate 110 mcg, and 1 subject who received salmeterol.
In another 12-week trial in adult and adolescent subjects with asthma, fluticasone propionate and salmeterol HFA 230 mcg/21 mcg (2 inhalations twice daily) was compared with ADVAIR DISKUS 500 mcg/50 mcg (1 inhalation twice daily) and fluticasone propionate CFC inhalation aerosol 220 mcg (2 inhalations twice daily) [see Clinical Studies (14.1)]. The geometric mean ratio of 24‑hour urinary cortisol excretion at week 12 compared with baseline was 0.9 for all 3 treatment groups.
The effect of 21 days of treatment with fluticasone propionate and salmeterol HFA 45 mcg/21 mcg (2 inhalations twice daily with or without a spacer) or ADVAIR DISKUS 100 mcg/50 mcg (1 inhalation twice daily) on serum cortisol was evaluated in 31 children aged 4 to 11 years with mild asthma. There were reductions in serum cortisol from baseline in all treatment groups (14%, 22%, and 13% for fluticasone propionate and salmeterol HFA, fluticasone propionate and salmeterol HFA with spacer, and ADVAIR DISKUS, respectively).
Other Fluticasone Propionate Products
Subjects with Asthma: Hypothalamic-Pituitary-Adrenal Axis Effects: In clinical trials with fluticasone propionate inhalation powder using dosages up to and including 250 mcg twice daily, occasional abnormal short cosyntropin tests (peak serum cortisol <18 mcg/dL assessed by radioimmunoassay) were noted both in subjects receiving fluticasone propionate and in subjects receiving placebo. The incidence of abnormal tests at 500 mcg twice daily was greater than placebo. In a 2-year trial carried out with the DISKHALER inhalation device in 64 subjects with mild, persistent asthma (mean FEV1 91% of predicted) randomized to fluticasone propionate 500 mcg twice daily or placebo, no subject receiving fluticasone propionate had an abnormal response to 6-hour cosyntropin infusion (peak serum cortisol <18 mcg/dL). With a peak cortisol threshold of <35 mcg/dL, 1 subject receiving fluticasone propionate (4%) had an abnormal response at 1 year; repeat testing at 18 months and 2 years was normal. Another subject receiving fluticasone propionate (5%) had an abnormal response at 2 years. No subject on placebo had an abnormal response at 1 or 2 years.
Other Salmeterol Xinafoate Products
Subjects with Asthma: Cardiovascular Effects: Inhaled salmeterol, like other beta-adrenergic agonist drugs, can produce dose-related cardiovascular effects and effects on blood glucose and/or serum potassium [see Warnings and Precautions (5.12, 5.18)]. The cardiovascular effects (heart rate, blood pressure) associated with salmeterol inhalation aerosol occur with similar frequency, and are of similar type and severity, as those noted following albuterol administration.
The effects of rising inhaled doses of salmeterol and standard inhaled doses of albuterol were studied in volunteers and in subjects with asthma. Salmeterol doses up to 84 mcg administered as inhalation aerosol resulted in heart rate increases of 3 to 16 beats/min, about the same as albuterol dosed at 180 mcg by inhalation aerosol (4 to 10 beats/min). In 2 double‑blind asthma trials, subjects receiving either 42 mcg of salmeterol inhalation aerosol twice daily (n = 81) or 180 mcg of albuterol inhalation aerosol 4 times daily (n = 80) underwent continuous electrocardiographic monitoring during four 24-hour periods; no clinically significant dysrhythmias were noted.
Concomitant Use of Fluticasone Propionate and Salmeterol HFA with Other Respiratory Medicines
Short-acting Beta2-agonists: In three 12-week U.S. clinical trials, the mean daily need for additional beta2-agonist use in 277 subjects receiving fluticasone propionate and salmeterol HFA was approximately 1.2 inhalations/day and ranged from 0 to 9 inhalations/day. Two percent (2%) of subjects receiving fluticasone propionate and salmeterol HFA in these trials averaged 6 or more inhalations per day over the course of the 12-week trials. No increase in frequency of cardiovascular adverse events was observed among subjects who averaged 6 or more inhalations per day.
Methylxanthines: The concurrent use of intravenously or orally administered methylxanthines (e.g., aminophylline, theophylline) by subjects receiving fluticasone propionate and salmeterol HFA has not been completely evaluated. In five 12-week clinical trials (3 U.S. and 2 non-U.S.), 45 subjects receiving fluticasone propionate and salmeterol HFA 45 mcg/21 mcg, fluticasone propionate and salmeterol HFA 115 mcg/21 mcg, or fluticasone propionate and salmeterol HFA 230 mcg/21 mcg twice daily concurrently with a theophylline product had adverse event rates similar to those in 577 subjects receiving fluticasone propionate and salmeterol HFA without theophylline.
Fluticasone Propionate Nasal Spray: In subjects receiving fluticasone propionate and salmeterol HFA in three 12-week U.S. clinical trials, no difference in the profile of adverse events or HPA axis effects was noted between subjects receiving FLONASE (fluticasone propionate) Nasal Spray, 50 mcg concurrently (n = 89) and those who were not (n = 192).
Close12.3 Pharmacokinetics
Absorption
Fluticasone Propionate: Healthy Subjects: Fluticasone propionate acts locally in the lung; therefore, plasma levels do not predict therapeutic effect. Trials using oral dosing of labeled and unlabeled drug have demonstrated that the oral systemic bioavailability of fluticasone propionate is negligible (<1%), primarily due to incomplete absorption and presystemic metabolism in the gut and liver. In contrast, the majority of the fluticasone propionate delivered to the lung is systemically absorbed.
Three (3) single-dose, placebo-controlled, crossover trials were conducted in healthy subjects: (1) a trial using 4 inhalations of fluticasone propionate and salmeterol HFA 230 mcg/21 mcg, salmeterol CFC inhalation aerosol 21 mcg, or fluticasone propionate CFC inhalation aerosol 220 mcg, (2) a trial using 8 inhalations of fluticasone propionate and salmeterol HFA 45 mcg/21 mcg, fluticasone propionate and salmeterol HFA 115 mcg/21 mcg, or fluticasone propionate and salmeterol HFA 230 mcg/21 mcg, and (3) a trial using 4 inhalations of fluticasone propionate and salmeterol HFA 230 mcg/21 mcg; 2 inhalations of ADVAIR DISKUS 500 mcg/50 mcg; 4 inhalations of fluticasone propionate CFC inhalation aerosol 220 mcg; or 1,010 mcg of fluticasone propionate given intravenously. Peak plasma concentrations of fluticasone propionate were achieved in 0.33 to 1.5 hours and those of salmeterol were achieved in 5 to 10 minutes.
Peak plasma concentrations of fluticasone propionate (N = 20 subjects) following 8 inhalations of fluticasone propionate and salmeterol HFA 45 mcg/21 mcg, fluticasone propionate and salmeterol HFA 115 mcg/21 mcg, and fluticasone propionate and salmeterol HFA 230 mcg/21 mcg averaged 41, 108, and 173 pg/mL, respectively.
Systemic exposure (N = 20 subjects) from 4 inhalations of fluticasone propionate and salmeterol HFA 230 mcg/21 mcg was 53% of the value from the individual inhaler for fluticasone propionate CFC inhalation aerosol and 42% of the value from the individual inhaler for salmeterol CFC inhalation aerosol. Peak plasma concentrations from fluticasone propionate and salmeterol HFA for fluticasone propionate (86 versus 120 pg/mL) and salmeterol (170 versus 510 pg/mL) were significantly lower compared with individual inhalers.
In 15 healthy subjects, systemic exposure to fluticasone propionate from 4 inhalations of fluticasone propionate and salmeterol HFA 230 mcg/21 mcg (920/84 mcg) and 2 inhalations of ADVAIR DISKUS 500 mcg/50 mcg (1,000/100 mcg) was similar between the 2 inhalers (i.e., 799 versus 832 pg•h/mL, respectively), but approximately half the systemic exposure from 4 inhalations of fluticasone propionate CFC inhalation aerosol 220 mcg (880 mcg, AUC = 1,543 pg•h/mL). Similar results were observed for peak fluticasone propionate plasma concentrations (186 and 182 pg/mL from fluticasone propionate and salmeterol HFA and ADVAIR DISKUS, respectively, and 307 pg/mL from the fluticasone propionate CFC inhalation aerosol). Absolute bioavailability of fluticasone propionate was 5.3% and 5.5% following administration of fluticasone propionate and salmeterol HFA and ADVAIR DISKUS, respectively.
Subjects with Asthma: A double-blind crossover trial was conducted in 13 adult subjects with asthma to evaluate the steady-state pharmacokinetics of fluticasone propionate and salmeterol following administration of 2 inhalations of fluticasone propionate and salmeterol HFA 115 mcg/21 mcg twice daily or 1 inhalation of ADVAIR DISKUS 250 mcg/50 mcg twice daily for 4 weeks. Systemic exposure (AUC) to fluticasone propionate was similar for fluticasone propionate and salmeterol HFA (274 pg•h/mL [95% CI: 150, 502]) and ADVAIR DISKUS (338 pg•h/mL [95% CI: 197, 581]).
The effect of 21 days of treatment with fluticasone propionate and salmeterol HFA 45 mcg/21 mcg (2 inhalations twice daily with or without a spacer) or ADVAIR DISKUS 100 mcg/50 mcg (1 inhalation twice daily) was evaluated in a trial of 31 children aged 4 to 11 years with mild asthma. Systemic exposure to fluticasone propionate was similar with ADVAIR DISKUS and fluticasone propionate and salmeterol HFA with a spacer (138 pg•h/mL [95% CI: 69, 273] and 107 pg•h/mL [95% CI: 46, 252], respectively) and lower with fluticasone propionate and salmeterol HFA without a spacer (24 pg•h/mL [95% CI: 10, 60]).
Salmeterol Xinafoate: Healthy Subjects: Salmeterol xinafoate, an ionic salt, dissociates in solution so that the salmeterol and 1-hydroxy-2-naphthoic acid (xinafoate) moieties are absorbed, distributed, metabolized, and eliminated independently. Salmeterol acts locally in the lung; therefore, plasma levels do not predict therapeutic effect.
Peak plasma concentrations of salmeterol (N = 20 subjects) following 8 inhalations of fluticasone propionate and salmeterol HFA 45 mcg/21 mcg, fluticasone propionate and salmeterol HFA 115 mcg/21 mcg, and fluticasone propionate and salmeterol HFA 230 mcg/21 mcg ranged from 220 to 470 pg/mL.
In 15 healthy subjects receiving fluticasone propionate and salmeterol HFA 230 mcg/21 mcg (920/84 mcg) and ADVAIR DISKUS 500 mcg/50 mcg (1,000/100 mcg), systemic exposure to salmeterol was higher (317 versus 169 pg•h/mL) and peak salmeterol concentrations were lower (196 versus 223 pg/mL) following fluticasone propionate and salmeterol HFA compared with ADVAIR DISKUS, although pharmacodynamic results were comparable.
Subjects with Asthma: Because of the small therapeutic dose, systemic levels of salmeterol are low or undetectable after inhalation of recommended dosages (42 mcg of salmeterol inhalation aerosol twice daily). Following chronic administration of an inhaled dose of 42 mcg of salmeterol inhalation aerosol twice daily, salmeterol was detected in plasma within 5 to 10 minutes in 6 subjects with asthma; plasma concentrations were very low, with mean peak concentrations of 150 pg/mL at 20 minutes and no accumulation with repeated doses.
A double-blind crossover trial was conducted in 13 adult subjects with asthma to evaluate the steady-state pharmacokinetics of fluticasone propionate and salmeterol following administration of 2 inhalations of fluticasone propionate and salmeterol HFA 115 mcg/21 mcg twice daily or 1 inhalation of ADVAIR DISKUS 250 mcg/50 mcg twice daily for 4 weeks. Systemic exposure to salmeterol was similar for fluticasone propionate and salmeterol HFA (53 pg•h/mL [95% CI: 17, 164]) and ADVAIR DISKUS (70 pg•h/mL [95% CI: 19, 254]).
The effect of 21 days of treatment with fluticasone propionate and salmeterol HFA 45 mcg/21 mcg (2 inhalations twice daily with or without a spacer) or ADVAIR DISKUS 100 mcg/50 mcg (1 inhalation twice daily) was evaluated in 31 children aged 4 to 11 years with mild asthma. Systemic exposure to salmeterol was similar for fluticasone propionate and salmeterol HFA, fluticasone propionate and salmeterol HFA with spacer, and ADVAIR DISKUS (126 pg•h/mL [95% CI: 70, 225], 103 pg•h/mL [95% CI: 54, 200], and 110 pg•h/mL [95% CI: 55, 219], respectively).
Distribution
Fluticasone Propionate: Following intravenous administration, the initial disposition phase for fluticasone propionate was rapid and consistent with its high lipid solubility and tissue binding. The volume of distribution averaged 4.2 L/kg.
The percentage of fluticasone propionate bound to human plasma proteins averages 99%. Fluticasone propionate is weakly and reversibly bound to erythrocytes and is not significantly bound to human transcortin.
Salmeterol: The percentage of salmeterol bound to human plasma proteins averages 96% in vitro over the concentration range of 8 to 7,722 ng of salmeterol base per milliliter, much higher concentrations than those achieved following therapeutic doses of salmeterol.
Elimination
Fluticasone Propionate: Following intravenous dosing, fluticasone propionate showed polyexponential kinetics and had a terminal elimination half-life of approximately 7.8 hours. The total clearance of fluticasone propionate is high (average, 1,093 mL/min), with renal clearance accounting for <0.02% of the total. Less than 5% of a radiolabeled oral dose was excreted in the urine as metabolites, with the remainder excreted in the feces as parent drug and metabolites. Terminal half-life estimates of fluticasone propionate for fluticasone propionate and salmeterol HFA, ADVAIR DISKUS, and fluticasone propionate CFC inhalation aerosol were similar and averaged 5.6 hours.
Salmeterol: In 2 healthy adult subjects who received 1 mg of radiolabeled salmeterol (as salmeterol xinafoate) orally, approximately 25% and 60% of the radiolabeled salmeterol was eliminated in urine and feces, respectively, over a period of 7 days. The terminal elimination half-life was about 5.5 hours (1 volunteer only).
The xinafoate moiety has no apparent pharmacologic activity. The xinafoate moiety is highly protein bound (>99%) and has a long elimination half-life of 11 days. No terminal half-life estimates were calculated for salmeterol following administration of fluticasone propionate and salmeterol HFA.
Metabolism: Fluticasone Propionate: The only circulating metabolite detected in man is the 17β-carboxylic acid derivative of fluticasone propionate, which is formed through the CYP3A4 pathway. This metabolite had less affinity (approximately 1/2,000) than the parent drug for the glucocorticoid receptor of human lung cytosol in vitro and negligible pharmacological activity in animal studies. Other metabolites detected in vitro using cultured human hepatoma cells have not been detected in man.
Salmeterol: Salmeterol base is extensively metabolized by hydroxylation, with subsequent elimination predominantly in the feces. No significant amount of unchanged salmeterol base was detected in either urine or feces.
An in vitro study using human liver microsomes showed that salmeterol is extensively metabolized to α-hydroxysalmeterol (aliphatic oxidation) by CYP3A4. Ketoconazole, a strong inhibitor of CYP3A4, essentially completely inhibited the formation of α-hydroxysalmeterol in vitro.
Specific Populations
A population pharmacokinetic analysis was performed for fluticasone propionate and salmeterol utilizing data from 9 controlled clinical trials that included 350 subjects with asthma aged 4 to 77 years who received treatment with ADVAIR DISKUS, fluticasone propionate and salmeterol HFA, fluticasone propionate inhalation powder (FLOVENT DISKUS), HFA-propelled fluticasone propionate inhalation aerosol (FLOVENT HFA), or CFC-propelled fluticasone propionate inhalation aerosol. The population pharmacokinetic analyses for fluticasone propionate and salmeterol showed no clinically relevant effects of age, gender, race, body weight, body mass index, or percent of predicted FEV1 on apparent clearance and apparent volume of distribution.
Patients with Hepatic and Renal Impairment: Formal pharmacokinetic studies using fluticasone propionate and salmeterol HFA have not been conducted in patients with hepatic or renal impairment. However, since both fluticasone propionate and salmeterol are predominantly cleared by hepatic metabolism, impairment of liver function may lead to accumulation of fluticasone propionate and salmeterol in plasma. Therefore, patients with hepatic disease should be closely monitored.
Drug Interaction Studies
In the repeat- and single-dose trials, there was no evidence of significant drug interaction in systemic exposure between fluticasone propionate and salmeterol when given alone or in combination via the DISKUS. Similar definitive studies have not been performed with fluticasone propionate and salmeterol HFA. The population pharmacokinetic analysis from 9 controlled clinical trials in 350 subjects with asthma showed no significant effects on fluticasone propionate or salmeterol pharmacokinetics following co-administration with beta2-agonists, corticosteroids, antihistamines, or theophyllines.
Inhibitors of Cytochrome P450 3A4: Ritonavir: Fluticasone Propionate: Fluticasone propionate is a substrate of CYP3A4. Coadministration of fluticasone propionate and the strong CYP3A4 inhibitor ritonavir is not recommended based upon a multiple-dose, crossover drug interaction trial in 18 healthy subjects. Fluticasone propionate aqueous nasal spray (200 mcg once daily) was coadministered for 7 days with ritonavir (100 mg twice daily). Plasma fluticasone propionate concentrations following fluticasone propionate aqueous nasal spray alone were undetectable (<10 pg/mL) in most subjects, and when concentrations were detectable peak levels (Cmax) averaged 11.9 pg/mL (range: 10.8 to 14.1 pg/mL) and AUC(0-τ) averaged 8.43 pg•h/mL (range: 4.2 to 18.8 pg•h/mL). Fluticasone propionate Cmax and AUC(0-τ) increased to 318 pg/mL (range: 110 to 648 pg/mL) and 3,102.6 pg•h/mL (range: 1,207.1 to 5,662.0 pg•h/mL), respectively, after coadministration of ritonavir with fluticasone propionate aqueous nasal spray. This significant increase in plasma fluticasone propionate exposure resulted in a significant decrease (86%) in serum cortisol AUC.
Ketoconazole: Fluticasone Propionate: In a placebo-controlled crossover trial in 8 healthy adult volunteers, coadministration of a single dose of orally inhaled fluticasone propionate (1,000 mcg) with multiple doses of ketoconazole (200 mg) to steady state resulted in increased plasma fluticasone propionate exposure, a reduction in plasma cortisol AUC, and no effect on urinary excretion of cortisol.
Salmeterol: In a placebo-controlled, crossover drug interaction trial in 20 healthy male and female subjects, coadministration of salmeterol (50 mcg twice daily) and the strong CYP3A4 inhibitor ketoconazole (400 mg once daily) for 7 days resulted in a significant increase in plasma salmeterol exposure as determined by a 16-fold increase in AUC (ratio with and without ketoconazole 15.76 [90% CI: 10.66, 23.31]) mainly due to increased bioavailability of the swallowed portion of the dose. Peak plasma salmeterol concentrations were increased by 1.4-fold (90% CI: 1.23, 1.68). Three (3) out of 20 subjects (15%) were withdrawn from salmeterol and ketoconazole coadministration due to beta-agonist–mediated systemic effects (2 with QTc prolongation and 1 with palpitations and sinus tachycardia). Coadministration of salmeterol and ketoconazole did not result in a clinically significant effect on mean heart rate, mean blood potassium, or mean blood glucose. Although there was no statistical effect on the mean QTc, coadministration of salmeterol and ketoconazole was associated with more frequent increases in QTc duration compared with salmeterol and placebo administration.
Erythromycin: Fluticasone Propionate: In a multiple-dose drug interaction trial, coadministration of orally inhaled fluticasone propionate (500 mcg twice daily) and erythromycin (333 mg 3 times daily) did not affect fluticasone propionate pharmacokinetics.
Salmeterol: In a repeat-dose trial in 13 healthy subjects, concomitant administration of erythromycin (a moderate CYP3A4 inhibitor) and salmeterol inhalation aerosol resulted in a 40% increase in salmeterol Cmax at steady state (ratio with and without erythromycin 1.4 [90% CI: 0.96, 2.03], P = 0.12), a 3.6-beat/min increase in heart rate ([95% CI: 0.19, 7.03], P<0.04), a 5.8-msec increase in QTc interval ([95% CI: -6.14, 17.77], P = 0.34), and no change in plasma potassium.
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Fluticasone Propionate - Fluticasone propionate demonstrated no tumorigenic potential in mice at oral doses up to 1,000 mcg/kg ...
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Fluticasone Propionate
Fluticasone propionate demonstrated no tumorigenic potential in mice at oral doses up to 1,000 mcg/kg (approximately 5 times the MRHDID on a mcg/m2 basis) for 78 weeks or in rats at inhalation doses up to 57 mcg/kg (approximately 0.5 times the MRHDID on a mcg/m2 basis) for 104 weeks.
Fluticasone propionate did not induce gene mutation in prokaryotic or eukaryotic cells in vitro. No significant clastogenic effect was seen in cultured human peripheral lymphocytes in vitro or in the in vivo mouse micronucleus test.
Fertility and reproductive performance were unaffected in male and female rats at subcutaneous doses up to 50 mcg/kg (approximately 0.5 times the MRHDID for adults on a mcg/m2 basis).
Salmeterol
In an 18-month carcinogenicity study in CD-mice, salmeterol at oral doses of 1,400 mcg/kg and above (approximately 10 times the MRHDID based on comparison of the plasma AUCs) caused a dose-related increase in the incidence of smooth muscle hyperplasia, cystic glandular hyperplasia, leiomyomas of the uterus, and ovarian cysts. No tumors were seen at 200 mcg/kg (approximately 2 times the MRHDID for adults based on comparison of the AUCs).
In a 24-month oral and inhalation carcinogenicity study in Sprague Dawley rats, salmeterol caused a dose-related increase in the incidence of mesovarian leiomyomas and ovarian cysts at doses of 680 mcg/kg and above (approximately 80 times the MRHDID on a mcg/m2 basis). No tumors were seen at 210 mcg/kg (approximately 25 times the MRHDID on a mcg/m2 basis). These findings in rodents are similar to those reported previously for other beta-adrenergic agonist drugs. The relevance of these findings to human use is unknown.
Salmeterol produced no detectable or reproducible increases in microbial and mammalian gene mutation in vitro. No clastogenic activity occurred in vitro in human lymphocytes or in vivo in a rat micronucleus test.
Fertility and reproductive performance were unaffected in male and female rats at oral doses up to 2,000 mcg/kg (approximately 230 times the MRHDID for adults on a mcg/m2 basis).
Close13.2 Animal Toxicology and/or Pharmacology
Studies in laboratory animals (minipigs, rodents, and dogs) have demonstrated the occurrence of cardiac arrhythmias and sudden death (with histologic evidence of myocardial necrosis) when beta-agonists and methylxanthines are administered concurrently. The clinical relevance of these findings is unknown.
-
14 CLINICAL STUDIESFluticasone propionate and salmeterol HFA has been studied in subjects with asthma aged 12 years and older. Fluticasone propionate and salmeterol HFA has not been studied in subjects younger than ...
Fluticasone propionate and salmeterol HFA has been studied in subjects with asthma aged 12 years and older. Fluticasone propionate and salmeterol HFA has not been studied in subjects younger than 12 years or in subjects with COPD. In clinical trials comparing fluticasone propionate and salmeterol HFA with its individual components, improvements in most efficacy endpoints were greater with fluticasone propionate and salmeterol HFA than with the use of either fluticasone propionate or salmeterol alone. In addition, clinical trials showed comparable results between fluticasone propionate and salmeterol HFA and ADVAIR DISKUS.
14.1 Trials Comparing Fluticasone Propionate and Salmeterol HFA with Fluticasone Propionate Alone or Salmeterol Alone
Four (4) double-blind, parallel-group clinical trials were conducted with fluticasone propionate and salmeterol HFA in 1,517 adult and adolescent subjects (aged 12 years and older, mean baseline FEV1 65% to 75% of predicted normal) with asthma that was not optimally controlled on their current therapy. All metered-dose inhaler treatments were inhalation aerosols given as 2 inhalations twice daily, and other maintenance therapies were discontinued.
Trial 1: Clinical Trial with Fluticasone Propionate and Salmeterol HFA 45 mcg/21 mcg
This placebo-controlled, 12-week, U.S. trial compared fluticasone propionate and salmeterol HFA 45 mcg/21 mcg with fluticasone propionate CFC inhalation aerosol 44 mcg or salmeterol CFC inhalation aerosol 21 mcg, each given as 2 inhalations twice daily. The primary efficacy endpoints were predose FEV1 and withdrawals due to worsening asthma. This trial was stratified according to baseline asthma therapy: subjects using beta-agonists (albuterol alone [n = 142], salmeterol [n = 84], or ICS [n = 134] [daily doses of beclomethasone dipropionate 252 to 336 mcg; budesonide 400 to 600 mcg; flunisolide 1,000 mcg; fluticasone propionate inhalation aerosol 176 mcg; fluticasone propionate inhalation powder 200 mcg; or triamcinolone acetonide 600 to 800 mcg]). Baseline FEV1 measurements were similar across treatments: fluticasone propionate and salmeterol HFA 45 mcg/21 mcg, 2.29 L; fluticasone propionate 44 mcg, 2.20 L; salmeterol, 2.33 L; and placebo, 2.27 L.
Predefined withdrawal criteria for lack of efficacy, an indicator of worsening asthma, were utilized for this placebo-controlled trial. Worsening asthma was defined as a clinically important decrease in FEV1 or PEF, increase in use of VENTOLIN (albuterol, USP) Inhalation Aerosol, increase in night awakenings due to asthma, emergency intervention or hospitalization due to asthma, or requirement for asthma medicine not allowed by the protocol. As shown in Table 3, statistically significantly fewer subjects receiving fluticasone propionate and salmeterol HFA 45 mcg/21 mcg were withdrawn due to worsening asthma compared with salmeterol and placebo. Fewer subjects receiving fluticasone propionate and salmeterol HFA 45 mcg/21 mcg were withdrawn due to worsening asthma compared with fluticasone propionate 44 mcg; however, the difference was not statistically significant.
Table 3. Percent of Subjects Withdrawn due to Worsening Asthma in Subjects Previously Treated with Beta2-agonists (Albuterol or Salmeterol) or Inhaled Corticosteroids (Trial 1) Fluticasone Propionate and Salmeterol HFA 45 mcg/21 mcg
(n = 92)
Fluticasone Propionate CFC Inhalation Aerosol
44 mcg
(n = 89)
Salmeterol CFC Inhalation Aerosol
21 mcg
(n = 92)
Placebo HFA Inhalation Aerosol
(n = 87)
2%
8%
25%
28%
The FEV1 results are displayed in Figure 1. Because this trial used predetermined criteria for worsening asthma, which caused more subjects in the placebo group to be withdrawn, FEV1 results at Endpoint (last available FEV1 result) are also provided. Subjects receiving fluticasone propionate and salmeterol HFA 45 mcg/21 mcg had significantly greater improvements in FEV1 (0.58 L, 27%) compared with fluticasone propionate 44 mcg (0.36 L, 18%), salmeterol (0.25 L, 12%), and placebo (0.14 L, 5%). These improvements in FEV1 with fluticasone propionate and salmeterol HFA 45 mcg/21 mcg were achieved regardless of baseline asthma therapy (albuterol alone, salmeterol, or ICS).
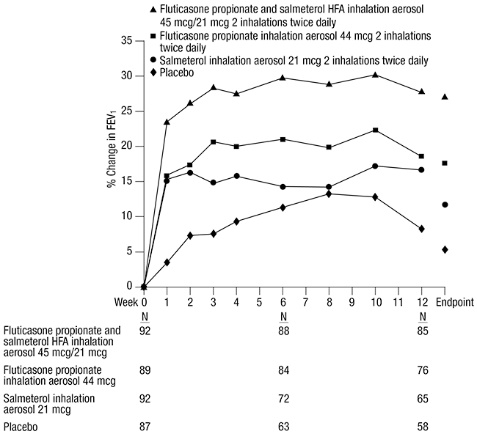
Figure 1. Mean Percent Change from Baseline in FEV1 in Subjects Previously Treated with Either Beta2-agonists (Albuterol or Salmeterol) or Inhaled Corticosteroids (Trial 1)
The effect of fluticasone propionate and salmeterol HFA 45 mcg/21 mcg on the secondary efficacy parameters, including morning and evening PEF, usage of VENTOLIN Inhalation Aerosol, and asthma symptoms over 24 hours on a scale of 0 to 5 is shown in Table 4.
Table 4. Secondary Efficacy Variable Results for Subjects Previously Treated with Beta2agonists (Albuterol or Salmeterol) or Inhaled Corticosteroids (Trial 1) a Change from baseline = change from baseline at Endpoint (last available data). Efficacy Variablea
Fluticasone Propionate and Salmeterol HFA
45 mcg/21 mcg
(n = 92)
Fluticasone Propionate CFC Inhalation Aerosol
44 mcg
(n = 89)
Salmeterol CFC Inhalation Aerosol
21 mcg
(n = 92)
Placebo HFA Inhalation Aerosol
(n = 87)
AM PEF (L/min)
- Baseline
377
369
381
382
- Change from baseline
58
27
25
1
PM PEF (L/min)
- Baseline
397
387
402
407
- Change from baseline
48
20
16
3
Use of VENTOLIN Inhalation Aerosol (inhalations/day)
- Baseline
3.1
2.4
2.7
2.7
- Change from baseline
-2.1
-0.4
-0.8
0.2
Asthma symptom score/day
- Baseline
1.8
1.6
1.7
1.7
- Change from baseline
-1.0
-0.3
-0.4
0
The subjective impact of asthma on subjects’ perception of health was evaluated through use of an instrument called the Asthma Quality of Life Questionnaire (AQLQ) (based on a 7-point scale where 1 = maximum impairment and 7 = none). Subjects receiving fluticasone propionate and salmeterol HFA 45 mcg/21 mcg had clinically meaningful improvements in overall asthma-specific quality of life as defined by a difference between groups of ≥0.5 points in change from baseline AQLQ scores (difference in AQLQ score of 1.14 [95% CI: 0.85, 1.44] compared with placebo).
Trial 2: Clinical Trial with Fluticasone Propionate and Salmeterol HFA 45 mcg/21 mcg
This active-controlled, 12-week, U.S. trial compared fluticasone propionate and salmeterol HFA 45 mcg/21 mcg with fluticasone propionate CFC inhalation aerosol 44 mcg and salmeterol CFC inhalation aerosol 21 mcg, each given as 2 inhalations twice daily, in 283 subjects using as-needed albuterol alone. The primary efficacy endpoint was predose FEV1. Baseline FEV1 measurements were similar across treatments: fluticasone propionate and salmeterol HFA 45 mcg/21 mcg, 2.37 L; fluticasone propionate 44 mcg, 2.31 L; and salmeterol, 2.34 L.
Efficacy results in this trial were similar to those observed in Trial 1. Subjects receiving fluticasone propionate and salmeterol HFA 45 mcg/21 mcg had significantly greater improvements in FEV1 (0.69 L, 33%) compared with fluticasone propionate 44 mcg (0.51 L, 25%) and salmeterol (0.47 L, 22%).
Trial 3: Clinical Trial with Fluticasone Propionate and Salmeterol HFA 115 mcg/21 mcg
This placebo-controlled, 12-week, U.S. trial compared fluticasone propionate and salmeterol HFA 115 mcg/21 mcg with fluticasone propionate CFC inhalation aerosol 110 mcg or salmeterol CFC inhalation aerosol 21 mcg, each given as 2 inhalations twice daily, in 365 subjects using ICS (daily doses of beclomethasone dipropionate 378 to 840 mcg; budesonide 800 to 1,200 mcg; flunisolide 1,250 to 2,000 mcg; fluticasone propionate inhalation aerosol 440 to 660 mcg; fluticasone propionate inhalation powder 400 to 600 mcg; or triamcinolone acetonide 900 to 1,600 mcg). The primary efficacy endpoints were predose FEV1 and withdrawals due to worsening asthma. Baseline FEV1 measurements were similar across treatments: fluticasone propionate and salmeterol HFA 115 mcg/21 mcg, 2.23 L; fluticasone propionate 110 mcg, 2.18 L; salmeterol, 2.22 L; and placebo, 2.17 L.
Efficacy results in this trial were similar to those observed in Trials 1 and 2. Subjects receiving fluticasone propionate and salmeterol HFA 115 mcg/21 mcg had significantly greater improvements in FEV1 (0.41 L, 20%) compared with fluticasone propionate 110 mcg (0.19 L, 9%), salmeterol (0.15 L, 8%), and placebo (-0.12 L, -6%). Significantly fewer subjects receiving fluticasone propionate and salmeterol HFA 115 mcg/21 mcg were withdrawn from this trial for worsening asthma (7%) compared with salmeterol (24%) and placebo (54%). Fewer subjects receiving fluticasone propionate and salmeterol HFA 115 mcg/21 mcg were withdrawn due to worsening asthma (7%) compared with fluticasone propionate 110 mcg (11%); however, the difference was not statistically significant.
Trial 4: Clinical Trial with Fluticasone Propionate and Salmeterol HFA 230 mcg/21 mcg
This active-controlled, 12-week, non-U.S. trial compared fluticasone propionate and salmeterol HFA 230 mcg/21 mcg with fluticasone propionate CFC inhalation aerosol 220 mcg, each given as 2 inhalations twice daily, and with ADVAIR DISKUS 500 mcg/50 mcg given as 1 inhalation twice daily in 509 subjects using ICS (daily doses of beclomethasone dipropionate CFC inhalation aerosol 1,500 to 2,000 mcg; budesonide 1,500 to 2,000 mcg; flunisolide 1,500 to 2,000 mcg; fluticasone propionate inhalation aerosol 660 to 880 mcg; or fluticasone propionate inhalation powder 750 to 1,000 mcg). The primary efficacy endpoint was morning PEF.
Baseline morning PEF measurements were similar across treatments: fluticasone propionate and salmeterol HFA 230 mcg/21 mcg, 327 L/min; ADVAIR DISKUS 500 mcg/50 mcg, 341 L/min; and fluticasone propionate 220 mcg, 345 L/min. As shown in Figure 2, morning PEF improved significantly with fluticasone propionate and salmeterol HFA 230 mcg/21 mcg compared with fluticasone propionate 220 mcg over the 12-week treatment period. Improvements in morning PEF observed with fluticasone propionate and salmeterol HFA 230 mcg/21 mcg were similar to improvements observed with ADVAIR DISKUS 500 mcg/50 mcg.
14.2 One-Year Safety Trial
Clinical Trial with Fluticasone Propionate and Salmeterol HFA 45 mcg/21 mcg, Fluticasone Propionate and Salmeterol HFA 115 mcg/21 mcg, and Fluticasone Propionate and Salmeterol HFA 230 mcg/21 mcg
This 1-year, open-label, non-U.S. trial evaluated the safety of fluticasone propionate and salmeterol HFA 45 mcg/21 mcg, fluticasone propionate and salmeterol HFA 115 mcg/21 mcg, and fluticasone propionate and salmeterol HFA 230 mcg/21 mcg given as 2 inhalations twice daily in 325 subjects. This trial was stratified into 3 groups according to baseline asthma therapy: subjects using short-acting beta2-agonists alone (n = 42), salmeterol (n = 91), or ICS (n = 277). Subjects treated with short-acting beta2-agonists alone, salmeterol, or low doses of ICS with or without concurrent salmeterol received fluticasone propionate and salmeterol HFA 45 mcg/21 mcg. Subjects treated with moderate doses of ICS with or without concurrent salmeterol received fluticasone propionate and salmeterol HFA 115 mcg/21 mcg. Subjects treated with high doses of ICS with or without concurrent salmeterol received fluticasone propionate and salmeterol HFA 230 mcg/21 mcg. Baseline FEV1 measurements ranged from 2.3 to 2.6 L.
Improvements in FEV1 (0.17 to 0.35 L at 4 weeks) were seen across all 3 treatments and were sustained throughout the 52-week treatment period. Few subjects (3%) were withdrawn due to worsening asthma over 1 year.
Close14.3 Onset of Action and Progression of Improvement in Control
The onset of action and progression of improvement in asthma control were evaluated in 2 placebo-controlled U.S. trials and 1 active-controlled U.S. trial. Following the first dose, the median time to onset of clinically significant bronchodilatation (≥15% improvement in FEV1) in most subjects was seen within 30 to 60 minutes. Maximum improvement in FEV1 occurred within 4 hours, and clinically significant improvement was maintained for 12 hours (Figure 3).
Following the initial dose, predose FEV1 relative to Day 1 baseline improved markedly over the first week of treatment and continued to improve over the 12 weeks of treatment in all 3 trials.
No diminution in the 12-hour bronchodilator effect was observed with either fluticasone propionate and salmeterol HFA 45 mcg/21 mcg (Figures 3 and 4) or fluticasone propionate and salmeterol HFA 230/21 as assessed by FEV1 following 12 weeks of therapy.
Figure 3. Percent Change in Serial 12-Hour FEV1 in Subjects Previously Using Either Beta2-agonists (Albuterol or Salmeterol) or Inhaled Corticosteroids (Trial 1)
First Treatment Day

Figure 4. Percent Change in Serial 12-Hour FEV1 in Subjects Previously Using Either Beta2-agonists (Albuterol or Salmeterol) or Inhaled Corticosteroids (Trial 1)
Last Treatment Day (Week 12)
Reduction in asthma symptoms and use of rescue VENTOLIN Inhalation Aerosol and improvement in morning and evening PEF also occurred within the first day of treatment with fluticasone propionate and salmeterol HFA and continued to improve over the 12 weeks of therapy in all 3 trials.
-
16 HOW SUPPLIED/STORAGE AND HANDLINGFluticasone Propionate and Salmeterol HFA is supplied in the following boxes of 1 as a pressurized aluminum canister fitted with a counter and supplied with a purple plastic actuator with a light ...
Fluticasone Propionate and Salmeterol HFA is supplied in the following boxes of 1 as a pressurized aluminum canister fitted with a counter and supplied with a purple plastic actuator with a light purple cap:
- •
- Fluticasone Propionate and Salmeterol HFA 45 mcg/21 mcg: 12-g canister containing 120 actuations (NDC 66993-086-96)
- •
- Fluticasone Propionate and Salmeterol HFA 115 mcg/21 mcg: 12-g canister containing 120 actuations (NDC 66993-087-96)
- •
- Fluticasone Propionate and Salmeterol HFA 230 mcg/21 mcg: 12-g canister containing 120 actuations (NDC 66993-088-96)
Each inhaler is packaged with a Patient Information leaflet.
The purple actuator supplied with Fluticasone Propionate and Salmeterol HFA should not be used with any other product canisters, and actuators from other products should not be used with a Fluticasone Propionate and Salmeterol HFA canister.
Counter
Fluticasone Propionate and Salmeterol HFA has a counter attached to the canister. The counter starts at 124 and counts down each time a spray is released. The correct amount of medication in each actuation cannot be assured after the counter reads 000, even though the canister is not completely empty and will continue to operate. The inhaler should be discarded when the counter reads 000.
Contents under Pressure
Do not puncture. Do not use or store near heat or open flame. Exposure to temperatures above 120°F may cause bursting. Never throw canister into fire or incinerator.
Storage
Store at room temperature between 68°F and 77°F (20°C and 25°C); excursions permitted from 59°F to 86°F (15°C to 30°C) [See USP Controlled Room Temperature]. Store the inhaler with the mouthpiece down. For best results, the inhaler should be at room temperature before use.
Close -
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use). Serious Asthma-Related Events - Inform patients with asthma that LABA when used alone ...
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Serious Asthma-Related Events
Inform patients with asthma that LABA when used alone increases the risk of asthma-related hospitalization or asthma-related death. Available data show that when ICS and LABA are used together, such as with Fluticasone Propionate and Salmeterol HFA, there is not a significant increase in the risk of these events. [See Warnings and Precautions (5.1).]
Not for Acute Symptoms
Inform patients that Fluticasone Propionate and Salmeterol HFA is not meant to relieve acute asthma symptoms and extra doses should not be used for that purpose. Advise patients to treat acute asthma symptoms with an inhaled, short-acting beta2-agonist such as albuterol. Provide patients with such medication and instruct them in how it should be used.
Instruct patients to seek medical attention immediately if they experience any of the following:
- •
- Decreasing effectiveness of inhaled, short-acting beta2-agonists
- •
- Need for more inhalations than usual of inhaled, short-acting beta2-agonists
- •
- Significant decrease in lung function as outlined by the physician
Tell patients they should not stop therapy with Fluticasone Propionate and Salmeterol HFA without physician/provider guidance since symptoms may recur after discontinuation. [See Warnings and Precautions (5.2).]
Do Not Use Additional Long-acting Beta2-agonists
Instruct patients not to use other LABA for asthma. [See Warnings and Precautions (5.3).]
Oropharyngeal Candidiasis
Inform patients that localized infections with Candida albicans occurred in the mouth and pharynx in some patients. If oropharyngeal candidiasis develops, treat it with appropriate local or systemic (i.e., oral) antifungal therapy while still continuing therapy with Fluticasone Propionate and Salmeterol HFA, but at times therapy with Fluticasone Propionate and Salmeterol HFA may need to be temporarily interrupted under close medical supervision. Advise patients to rinse the mouth with water without swallowing after inhalation to help reduce the risk of thrush. [See Warnings and Precautions (5.4).]
Pneumonia
Patients with COPD have a higher risk of pneumonia; instruct them to contact their healthcare providers if they develop symptoms of pneumonia. [See Warnings and Precautions (5.5).]
Immunosuppression and Risk of Infections
Warn patients who are on immunosuppressant doses of corticosteroids to avoid exposure to chickenpox or measles and, if exposed, to consult their physicians without delay. Inform patients of potential worsening of existing tuberculosis; fungal, bacterial, viral, or parasitic infections; or ocular herpes simplex. [See Warnings and Precautions (5.6).]
Hypercorticism and Adrenal Suppression
Advise patients that Fluticasone Propionate and Salmeterol HFA may cause systemic corticosteroid effects of hypercorticism and adrenal suppression. Additionally, inform patients that deaths due to adrenal insufficiency have occurred during and after transfer from systemic corticosteroids. Patients should taper slowly from systemic corticosteroids if transferring to Fluticasone Propionate and Salmeterol HFA. [See Warnings and Precautions (5.8).]
Hypersensitivity Reactions, including Anaphylaxis
Advise patients that immediate hypersensitivity reactions (e.g., urticaria, angioedema, rash, bronchospasm, hypotension), including anaphylaxis, may occur after administration of Fluticasone Propionate and Salmeterol HFA. Patients should discontinue Fluticasone Propionate and Salmeterol HFA if such reactions occur. [See Warnings and Precautions (5.11).]
Risks Associated with Beta-agonist Therapy
Inform patients of adverse effects associated with beta2-agonists, such as palpitations, chest pain, rapid heart rate, tremor, or nervousness. [See Warnings and Precautions (5.12).]
Reduction in Bone Mineral Density
Advise patients who are at an increased risk for decreased BMD that the use of corticosteroids may pose an additional risk. [See Warnings and Precautions (5.13).]
Reduced Growth Velocity
Inform patients that orally inhaled corticosteroids, including fluticasone propionate, may cause a reduction in growth velocity when administered to pediatric patients. Physicians should closely follow the growth of children and adolescents taking corticosteroids by any route. [See Warnings and Precautions (5.14).]
Glaucoma and Cataracts
Advise patients that long-term use of ICS may increase the risk of some eye problems (cataracts or glaucoma); consider regular eye examinations. [See Warnings and Precautions (5.15).]
Trademarks are owned by or licensed to the GSK group of companies.
Manufactured for:
Prasco Laboratories
Mason, OH 45040 USA
Manufactured by:
GlaxoSmithKline
Durham, NC 27701
ADH-PS:2PI
Close -
PATIENT PACKAGE INSERTPATIENT INFORMATION - Fluticasone Propionate and Salmeterol HFA - inhalation aerosol - for oral inhalation use - What is Fluticasone Propionate and Salmeterol HFA? • Fluticasone ...
PATIENT INFORMATION
Fluticasone Propionate and Salmeterol HFA
inhalation aerosol
for oral inhalation use
What is Fluticasone Propionate and Salmeterol HFA?
- •
- Fluticasone Propionate and Salmeterol HFA combines the inhaled corticosteroid (ICS) medicine fluticasone propionate and the long-acting beta2-adrenergic agonist (LABA) medicine salmeterol.
- o
- ICS medicines such as fluticasone propionate help to decrease inflammation in the lungs. Inflammation in the lungs can lead to breathing problems.
- o
- LABA medicines such as salmeterol help the muscles around the airways in your lungs stay relaxed to prevent symptoms, such as wheezing, cough, chest tightness, and shortness of breath. These symptoms can happen when the muscles around the airways tighten. This makes it hard to breathe.
- •
- Fluticasone Propionate and Salmeterol HFA is not used to relieve sudden breathing problems and will not replace a rescue inhaler.
- •
- It is not known if Fluticasone Propionate and Salmeterol HFA is safe and effective in children younger than 12 years of age.
- •
- Fluticasone Propionate and Salmeterol HFA is used for asthma as follows:
- o
- Fluticasone Propionate and Salmeterol HFA is a prescription medicine used to control symptoms of asthma and to prevent symptoms such as wheezing in adults and adolescents aged 12 years and older.
- o
- Fluticasone Propionate and Salmeterol HFA contains salmeterol, the same medicine found in SEREVENT DISKUS (salmeterol xinafoate inhalation powder). LABA medicines such as salmeterol when used alone increase the risk of hospitalizations and death from asthma problems. Fluticasone Propionate and Salmeterol HFA contains an ICS and a LABA. When an ICS and LABA are used together, there is not a significant increased risk in hospitalizations and death from asthma problems.
- o
- Fluticasone Propionate and Salmeterol HFA is not for adults and adolescents with asthma who are well controlled with an asthma control medicine, such as a low to medium dose of an ICS medicine. Fluticasone Propionate and Salmeterol HFA is for adults and adolescents with asthma who need both an ICS and LABA medicine.
Do not use Fluticasone Propionate and Salmeterol HFA:
- •
- to relieve sudden breathing problems.
- •
- as a rescue inhaler.
- •
- if you are allergic to fluticasone propionate, salmeterol, or any of the ingredients in Fluticasone Propionate and Salmeterol HFA. See the end of this Patient Information for a complete list of ingredients in Fluticasone Propionate and Salmeterol HFA.
Before using Fluticasone Propionate and Salmeterol HFA, tell your healthcare provider about all of your medical conditions, including if you:
- •
- have heart problems.
- •
- have high blood pressure.
- •
- have seizures.
- •
- have thyroid problems.
- •
- have diabetes.
- •
- have liver problems.
- •
- have weak bones (osteoporosis).
- •
- have an immune system problem.
- •
- have or have had eye problems such as glaucoma, increased pressure in your eye, cataracts, or other changes in vision.
- •
- have any type of viral, bacterial, or fungal infection.
- •
- are exposed to chickenpox or measles.
- •
- are pregnant or plan to become pregnant. It is not known if Fluticasone Propionate and Salmeterol HFA may harm your unborn baby.
- •
- are breastfeeding. It is not known if the medicines in Fluticasone Propionate and Salmeterol HFA pass into your milk and if they can harm your baby.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Fluticasone Propionate and Salmeterol HFA and certain other medicines may interact with each other. This may cause serious side effects. Especially tell your healthcare provider if you take antifungal or anti-HIV medicines.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I use Fluticasone Propionate and Salmeterol HFA?
Read the step-by-step instructions for using Fluticasone Propionate and Salmeterol HFA at the end of this Patient Information.
- •
- Do not use Fluticasone Propionate and Salmeterol HFA unless your healthcare provider has taught you how to use the inhaler and you understand how to use it correctly.
- •
- Fluticasone Propionate and Salmeterol HFA comes in 3 different strengths. Your healthcare provider prescribed the strength that is best for you.
- •
- Use Fluticasone Propionate and Salmeterol HFA exactly as your healthcare provider tells you to use it. Do not use Fluticasone Propionate and Salmeterol HFA more often than prescribed.
- •
- Use 2 inhalations of Fluticasone Propionate and Salmeterol HFA 2 times each day. Use Fluticasone Propionate and Salmeterol HFA at the same time each day, about 12 hours apart.
- •
- If you miss a dose of Fluticasone Propionate and Salmeterol HFA, just skip that dose. Take your next dose at your usual time. Do not take 2 doses at 1 time.
- •
- If you take too much Fluticasone Propionate and Salmeterol HFA, call your healthcare provider or go to the nearest hospital emergency room right away if you have any unusual symptoms, such as worsening shortness of breath, chest pain, increased heart rate, or shakiness.
- •
- Do not use other medicines that contain a LABA for any reason. Ask your healthcare provider or pharmacist if any of your other medicines are LABA medicines.
- •
- Do not stop using Fluticasone Propionate and Salmeterol HFA, even if you are feeling better, unless your healthcare provider tells you to.
- •
- Fluticasone Propionate and Salmeterol HFA does not relieve sudden breathing problems. Always have a rescue inhaler with you to treat sudden symptoms. If you do not have a rescue inhaler, call your healthcare provider to have one prescribed for you.
- •
- Rinse your mouth with water without swallowing after each dose of Fluticasone Propionate and Salmeterol HFA. This will help lessen the chance of getting a yeast infection (thrush) in your mouth and throat.
- •
- Call your healthcare provider or get medical care right away if:
- o
- your breathing problems get worse.
- o
- you need to use your rescue inhaler more often than usual.
- o
- your rescue inhaler does not work as well to relieve your symptoms.
- o
- you need to use 4 or more inhalations of your rescue inhaler in 24 hours for 2 or more days in a row.
- o
- you use 1 whole canister of your rescue inhaler in 8 weeks.
- o
- your peak flow meter results decrease. Your healthcare provider will tell you the numbers that are right for you.
- o
- you have asthma and your symptoms do not improve after using Fluticasone Propionate and Salmeterol HFA regularly for 1 week.
What are the possible side effects of Fluticasone Propionate and Salmeterol HFA?
Fluticasone Propionate and Salmeterol HFA can cause serious side effects, including:
- •
- fungal infection in your mouth or throat (thrush). Rinse your mouth with water without swallowing after using Fluticasone Propionate and Salmeterol HFA to help reduce your chance of getting thrush.
- •
- pneumonia. Fluticasone Propionate and Salmeterol HFA contains the same medicine found in ADVAIR DISKUS (fluticasone propionate and salmeterol inhalation powder). ADVAIR DISKUS is used to treat people with asthma and people with chronic obstructive pulmonary disease (COPD). People with COPD have a higher chance of getting pneumonia. ADVAIR DISKUS may increase the chance of you getting pneumonia. It is not known if Fluticasone Propionate and Salmeterol HFA is safe and effective in people with COPD. Call your healthcare provider right away if you have any of the following symptoms:
- ○increase in mucus (sputum) production
- ○change in mucus color
- ○fever
- ○chills
- ○increased cough
- ○increased breathing problems
- •
- weakened immune system and increased chance of getting infections (immunosuppression).
- •
- reduced adrenal function (adrenal insufficiency). Adrenal insufficiency is a condition where the adrenal glands do not make enough steroid hormones. This can happen when you stop taking oral corticosteroid medicines (such as prednisone) and start taking a medicine containing an inhaled steroid (such as Fluticasone Propionate and Salmeterol HFA). During this transition period, when your body is under stress such as from fever, trauma (such as a car accident), infection, surgery, or worse COPD symptoms, adrenal insufficiency can get worse and may cause death.
- Symptoms of adrenal insufficiency include:
- ○feeling tired
- ○lack of energy
- ○weakness
- ○nausea and vomiting
- ○low blood pressure (hypotension)
- •
- sudden breathing problems immediately after inhaling your medicine. If you have sudden breathing problems immediately after inhaling your medicine, stop using Fluticasone Propionate and Salmeterol HFA and call your healthcare provider right away.
- •
- serious allergic reactions. Call your healthcare provider or get emergency medical care if you get any of the following symptoms of a serious allergic reaction:
- ○rash
- ○hives
- ○swelling of your face, mouth, and tongue
- ○breathing problems
- •
- effects on heart.
- ○increased blood pressure
- ○a fast or irregular heartbeat
- ○chest pain
- •
- effects on nervous system.
- ○tremor
- ○nervousnes
- •
- bone thinning or weakness (osteoporosis).
- •
- slowed growth in children. Your child’s growth should be checked regularly by the healthcare provider while using Fluticasone Propionate and Salmeterol HFA.
- •
- eye problems including glaucoma, increased pressure in your eye, cataracts, or other changes in vision. You should have regular eye exams while using Fluticasone Propionate and Salmeterol HFA.
- •
- changes in laboratory blood levels (sugar, potassium, certain types of white blood cells).
Common side effects of Fluticasone Propionate and Salmeterol HFA include:
-
- o
- upper respiratory tract infection
- o
- throat irritation
- o
- hoarseness and voice changes
- •
- headache
- •
- dizziness
- •
- nausea and vomiting
These are not all the possible side effects of Fluticasone Propionate and Salmeterol HFA.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store Fluticasone Propionate and Salmeterol HFA?
- •
- Store Fluticasone Propionate and Salmeterol HFA at room temperature between 68°F and 77°F (20°C and 25°C) with the mouthpiece down.
- •
- The contents of your Fluticasone Propionate and Salmeterol HFA are under pressure: Do not puncture. Do not use or store near heat or open flame. Temperatures above 120°F may cause the canister to burst.
- •
- Do not throw into fire or an incinerator.
- •
- Safely throw away Fluticasone Propionate and Salmeterol HFA in the trash when the counter reads 000.
Keep Fluticasone Propionate and Salmeterol HFA and all medicines out of the reach of children.
General information about the safe and effective use of Fluticasone Propionate and Salmeterol HFA.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Fluticasone Propionate and Salmeterol HFA for a condition for which it was not prescribed. Do not give Fluticasone Propionate and Salmeterol HFA to other people, even if they have the same symptoms that you have. It may harm them.
You can ask your healthcare provider or pharmacist for information about Fluticasone Propionate and Salmeterol HFA that was written for health professionals.
What are the ingredients in Fluticasone Propionate and Salmeterol HFA?
Active ingredients: fluticasone propionate, salmeterol xinafoate
Inactive ingredient: propellant HFA-134a
For more information about Fluticasone Propionate and Salmeterol HFA, call 1-866-525-0688.
Trademarks are owned by or licensed to the GSK group of companies.
Manufactured for:
Prasco Laboratories
Mason, OH 45040 USA
Manufactured by:
GlaxoSmithKline
Durham, NC 27701
ADH-PS:1PIL
- This Patient Information has been approved by the U.S. Food and Drug Administration Revised: August 2022
-
INSTRUCTIONS FOR USEINSTRUCTIONS FOR USE - Fluticasone Propionate and Salmeterol HFA - inhalation aerosol - for oral inhalation use - Your Fluticasone Propionate and Salmeterol HFA inhaler - Figure ...Close
-
PRINCIPAL DISPLAY PANELPRINCIPAL DISPLAY PANEL - NDC 66993-086-96 - Fluticasone Propionate and Salmeterol HFA Inhalation Aerosol - 45 mcg/21 mcg - PRASCO - For oral inhalation with Fluticasone Propionate and Salmeterol ...
PRINCIPAL DISPLAY PANEL
NDC 66993-086-96
Fluticasone Propionate and Salmeterol HFA Inhalation Aerosol
45 mcg/21 mcg
PRASCO
For oral inhalation with Fluticasone Propionate and Salmeterol HFA actuator only.
Contents: Each canister contains a microcrystalline suspension of fluticasone propionate and salmeterol xinafoate in propellant HFA-134a (1,1,1,2-tetrafluoroethane).
Each actuation delivers 45 mcg of fluticasone propionate and 30.45 mcg of salmeterol xinafoate equivalent to 21 mcg of salmeterol base from the mouthpiece.
See prescribing information for dosage information.
RX Only
120 Metered Actuations
Net Wt. 12 g
- 62000000083185 Rev. 10/22
-
PRINCIPAL DISPLAY PANELPRINCIPAL DISPLAY PANEL - NDC 66993-087-96 - Fluticasone Propionate and Salmeterol HFA Inhalation Aerosol - 115 mcg/21 mcg - PRASCO - For oral inhalation with Fluticasone Propionate and Salmeterol ...
PRINCIPAL DISPLAY PANEL
NDC 66993-087-96
Fluticasone Propionate and Salmeterol HFA Inhalation Aerosol
115 mcg/21 mcg
PRASCO
For oral inhalation with Fluticasone Propionate and Salmeterol HFA actuator only.
Contents: Each canister contains a microcrystalline suspension of fluticasone propionate and salmeterol xinafoate in propellant HFA-134a (1,1,1,2-tetrafluoroethane).
Each actuation delivers 115 mcg of fluticasone propionate and 30.45 mcg of salmeterol xinafoate equivalent to 21 mcg of salmeterol base from the mouthpiece.
See prescribing information for dosage information.
RX Only
120 Metered Actuations
Net Wt. 12 g
- 62000000083187 Rev. 10/22
-
PRINCIPAL DISPLAY PANELPRINCIPAL DISPLAY PANEL - NDC 66993-088-96 - Fluticasone Propionate and Salmeterol HFA Inhalation Aerosol - 230 mcg/21 mcg - PRASCO - For oral inhalation with Fluticasone Propionate and Salmeterol ...
PRINCIPAL DISPLAY PANEL
NDC 66993-088-96
Fluticasone Propionate and Salmeterol HFA Inhalation Aerosol
230 mcg/21 mcg
PRASCO
For oral inhalation with Fluticasone Propionate and Salmeterol HFA actuator only.
Contents: Each canister contains a microcrystalline suspension of fluticasone propionate and salmeterol xinafoate in propellant HFA-134a (1,1,1,2-tetrafluoroethane).
Each actuation delivers 230 mcg of fluticasone propionate and 30.45 mcg of salmeterol xinafoate equivalent to 21 mcg of salmeterol base from the mouthpiece.
See prescribing information for dosage information.
RX Only
120 Metered Actuations
Net Wt. 12 g
- 6200000083189 Rev. 10/22
-
INGREDIENTS AND APPEARANCEProduct Information
FLUTICASONE PROPIONATE AND SALMETEROL HFA fluticasone propionate and salmeterol xinafoate aerosol, metered Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:66993-086 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FLUTICASONE PROPIONATE (UNII: O2GMZ0LF5W) (FLUTICASONE - UNII:CUT2W21N7U) FLUTICASONE PROPIONATE 45 ug SALMETEROL XINAFOATE (UNII: 6EW8Q962A5) (SALMETEROL - UNII:2I4BC502BT) SALMETEROL 21 ug Inactive Ingredients Ingredient Name Strength NORFLURANE (UNII: DH9E53K1Y8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66993-086-96 1 in 1 CARTON 03/01/2023 1 120 in 1 INHALER; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA authorized generic NDA021254 03/01/2023 FLUTICASONE PROPIONATE AND SALMETEROL HFA fluticasone propionate and salmeterol xinafoate aerosol, metered Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:66993-087 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FLUTICASONE PROPIONATE (UNII: O2GMZ0LF5W) (FLUTICASONE - UNII:CUT2W21N7U) FLUTICASONE PROPIONATE 115 ug SALMETEROL XINAFOATE (UNII: 6EW8Q962A5) (SALMETEROL - UNII:2I4BC502BT) SALMETEROL 21 ug Inactive Ingredients Ingredient Name Strength NORFLURANE (UNII: DH9E53K1Y8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66993-087-96 1 in 1 CARTON 03/01/2023 1 120 in 1 INHALER; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA authorized generic NDA021254 03/01/2023 FLUTICASONE PROPIONATE AND SALMETEROL HFA fluticasone propionate and salmeterol xinafoate aerosol, metered Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:66993-088 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FLUTICASONE PROPIONATE (UNII: O2GMZ0LF5W) (FLUTICASONE - UNII:CUT2W21N7U) FLUTICASONE PROPIONATE 230 ug SALMETEROL XINAFOATE (UNII: 6EW8Q962A5) (SALMETEROL - UNII:2I4BC502BT) SALMETEROL 21 ug Inactive Ingredients Ingredient Name Strength NORFLURANE (UNII: DH9E53K1Y8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66993-088-96 1 in 1 CARTON 03/01/2023 1 120 in 1 INHALER; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA authorized generic NDA021254 03/01/2023 Labeler - Prasco Laboratories (065969375)
CloseRegistrant - GlaxoSmithKline LLC (167380711)
Find additional resources
(also available in the left menu)Safety
Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
View Labeling Archives for this drug
FLUTICASONE PROPIONATE AND SALMETEROL HFA- fluticasone propionate and salmeterol xinafoate aerosol, metered
Number of versions: 3
| Published Date (What is this?) | Version | Files |
|---|---|---|
| May 26, 2025 | 4 (current) | download |
| Jul 12, 2024 | 3 | download |
| Mar 3, 2023 | 2 | download |
RxNorm
FLUTICASONE PROPIONATE AND SALMETEROL HFA- fluticasone propionate and salmeterol xinafoate aerosol, metered
| RxCUI | RxNorm NAME | RxTTY | |
|---|---|---|---|
| 1 | 896236 | fluticasone propionate/salmeterol 45/21 MCG/INHAL Metered Dose Inhaler, 120 Actuations | PSN |
| 2 | 896236 | 120 ACTUAT fluticasone propionate 0.045 MG/ACTUAT / salmeterol 0.021 MG/ACTUAT Metered Dose Inhaler | SCD |
| 3 | 896236 | fluticasone propionate 0.045 MG / salmeterol 0.021 MG per ACTUAT Metered Dose Inhaler, 120 ACTUAT | SY |
| 4 | 896236 | fluticasone propionate 45 MCG / salmeterol 21 MCG (salmeterol xinafoate 30.45 MCG) per ACTUAT Metered Dose Inhaler, 120 ACTUAT | SY |
| 5 | 896244 | fluticasone propionate/salmeterol 115/21 MCG/INHAL Metered Dose Inhaler, 120 Actuations | PSN |
| 6 | 896244 | 120 ACTUAT fluticasone propionate 0.115 MG/ACTUAT / salmeterol 0.021 MG/ACTUAT Metered Dose Inhaler | SCD |
| 7 | 896244 | fluticasone propionate 0.115 MG / salmeterol 0.021 MG per ACTUAT Metered Dose Inhaler, 120 ACTUAT | SY |
| 8 | 896244 | fluticasone propionate 115 MCG / salmeterol 21 MCG (salmeterol xinafoate 30.45 MCG) per ACTUAT Metered Dose Inhaler, 120 ACTUAT | SY |
| 9 | 896272 | fluticasone propionate/salmeterol 230/21 MCG/INHAL Metered Dose Inhaler, 120 Actuations | PSN |
| 10 | 896272 | 120 ACTUAT fluticasone propionate 0.23 MG/ACTUAT / salmeterol 0.021 MG/ACTUAT Metered Dose Inhaler | SCD |
| 11 | 896272 | fluticasone propionate 0.23 MG / salmeterol 0.021 MG per ACTUAT Metered Dose Inhaler, 120 ACTUAT | SY |
| 12 | 896272 | fluticasone propionate 230 MCG / salmeterol 21 MCG (salmeterol xinafoate 30.45 MCG) per ACTUAT Metered Dose Inhaler, 120 ACTUAT | SY |
Get Label RSS Feed for this Drug
FLUTICASONE PROPIONATE AND SALMETEROL HFA- fluticasone propionate and salmeterol xinafoate aerosol, metered
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=666c5b12-a4f7-4c53-8261-4742b5e5a6df
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
FLUTICASONE PROPIONATE AND SALMETEROL HFA- fluticasone propionate and salmeterol xinafoate aerosol, metered
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 66993-086-96 |
| 2 | 66993-087-96 |
| 3 | 66993-088-96 |