Label: REPAGLINIDE tablet
- NDC Code(s): 65862-670-01, 65862-670-05, 65862-670-99, 65862-671-01, view more
- Packager: Aurobindo Pharma Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 14, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use REPAGLINIDE TABLETS safely and effectively. See full prescribing information for REPAGLINIDE TABLETS. REPAGLINIDE tablets, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGERepaglinide tablets are indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. Limitation of Use: Repaglinide tablets should not ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage and Administration - The recommended starting dose for patients whose HbA1c is less than 8% is 0.5 mg orally before each meal. For patients whose HbA1c is 8% or greater the ...
-
3 DOSAGE FORMS AND STRENGTHSRepaglinide tablets USP, 0.5 mg are white to off white, round, biconvex uncoated tablets, debossed with ‘H’ on one side and ‘10’ on other side. Repaglinide tablets USP, 1 mg are yellow ...
-
4 CONTRAINDICATIONSRepaglinide tablets are contraindicated in patients with: Concomitant use of gemfibrozil [see Drug Interactions (7)] Known hypersensitivity to repaglinide or any inactive ingredients
-
5 WARNINGS AND PRECAUTIONS5.1 Hypoglycemia - All glinides, including repaglinide, can cause hypoglycemia [see Adverse Reactions (6.1)]. Severe hypoglycemia can cause seizures, may be life-threatening, or cause death ...
-
6 ADVERSE REACTIONSThe following serious adverse reaction is also described elsewhere in the labeling: Hypoglycemia [see Warnings and Precautions (5.1)] 6.1 Clinical Trials Experience - Because clinical trials are ...
-
7 DRUG INTERACTIONSClinically Important Drug Interactions with Repaglinide - Table 3 includes a list of drugs with clinically important drug interactions when administered concomitantly with repaglinide and ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Limited available data from case reports and case series with repaglinide use in pregnant women have not identified a drug-associated risk of major birth ...
-
10 OVERDOSAGESevere hypoglycemic reactions with coma, seizure, or other neurological impairment may occur and constitute medical emergencies requiring immediate hospitalization. Hypoglycemic symptoms without ...
-
11 DESCRIPTIONRepaglinide is an oral blood glucose-lowering drug of the glinide class. Repaglinide, S(+)2-ethoxy-4(2((3-methyl-1-(2-(1-piperidinyl) phenyl)-butyl) amino)-2-oxoethyl) benzoic acid, is chemically ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Repaglinide lowers blood glucose levels by stimulating the release of insulin from the pancreas. This action is dependent upon functioning beta (ß) cells in the ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - In a 104-week carcinogenicity study in rats at doses up to 120 mg/kg/day, which is approximately 90 times clinical exposure on a mg/m2 ...
-
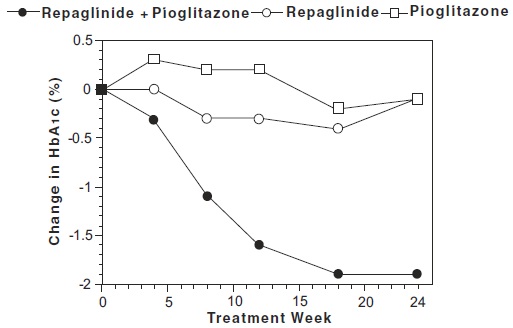
14 CLINICAL STUDIES14.1 Monotherapy Trials - A double-blind, placebo-controlled trial was carried out in 362 patients treated for 24 weeks. HbA1c for the repaglinide-treated groups (1 and 4 mg groups combined) at ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGRepaglinide Tablets USP, 0.5 mg are white to off white, round, biconvex uncoated tablets, debossed with ‘H’ on one side and ‘10’ on other side. Bottles of 100 NDC ...
-
17 PATIENT COUNSELING INFORMATIONHypoglycemia - Inform patients that repaglinide tablets can cause hypoglycemia and instruct patients and their caregivers on self-management procedures including glucose monitoring and ...
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 0.5 mg (100 Tablet Bottle)NDC 65862-670-01 - Repaglinide Tablets, USP - 0.5 mg - Rx only 100 Tablets - AUROBINDO
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 1 mg (100 Tablet Bottle)NDC 65862-671-01 - Rx only - Repaglinide Tablets, USP - 1 mg - AUROBINDO 100 Tablets
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 2 mg (100 Tablet Bottle)NDC 65862-672-01 - Rx only - Repaglinide Tablets, USP - 2 mg - AUROBINDO 100 Tablets
-
INGREDIENTS AND APPEARANCEProduct Information