Label: MEMANTINE HYDROCHLORIDE tablet, film coated
- NDC Code(s): 65862-652-03, 65862-652-10, 65862-652-60, 65862-652-78, view more
- Packager: Aurobindo Pharma Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 8, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use MEMANTINE HYDROCHLORIDE TABLETS safely and effectively. See full prescribing information for MEMANTINE HYDROCHLORIDE TABLETS ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEMemantine hydrochloride tablets are indicated for the treatment of moderate to severe dementia of the Alzheimer’s type.
-
2 DOSAGE AND ADMINISTRATIONThe recommended starting dose of memantine hydrochloride tablets is 5 mg once daily. The dose should be increased in 5 mg increments to 10 mg/day (5 mg twice daily), 15 mg/day (5 mg and 10 mg as ...
-
3 DOSAGE FORMS AND STRENGTHSMemantine hydrochloride 5 mg tablet: white to off-white, modified caplet shaped, film-coated tablets debossed with ‘Z’ on one side and ‘01’ on other side. Memantine hydrochloride 10 mg tablet ...
-
4 CONTRAINDICATIONSMemantine hydrochloride tablets are contraindicated in patients with known hypersensitivity to memantine hydrochloride or to any excipients used in the formulation.
-
5 WARNINGS AND PRECAUTIONS5.1 Genitourinary Conditions - Conditions that raise urine pH may decrease the urinary elimination of memantine resulting in increased plasma levels of memantine [see Drug Interactions ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Memantine hydrochloride was evaluated in eight double-blind placebo-controlled trials involving a total of 1862 dementia (Alzheimer’s disease, vascular dementia ...
-
7 DRUG INTERACTIONS7.1 Drugs that Make the Urine Alkaline - The clearance of memantine was reduced by about 80% under alkaline urine conditions at pH 8. Therefore, alterations of urine pH towards the alkaline ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no adequate data on the developmental risk associated with the use of memantine hydrochloride in pregnant women. Adverse developmental effects ...
-
10 OVERDOSAGESigns and symptoms most often accompanying memantine overdosage in clinical trials and from worldwide marketing experience, alone or in combination with other drugs and/or alcohol, include ...
-
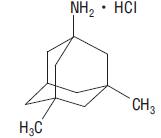
11 DESCRIPTIONMemantine hydrochloride is an orally active NMDA receptor antagonist. The chemical name for memantine hydrochloride is 1-amino-3,5-dimethyladamantane hydrochloride with the following structural ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Persistent activation of central nervous system N-methyl-D-aspartate (NMDA) receptors by the excitatory amino acid glutamate has been hypothesized to contribute to the ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - There was no evidence of carcinogenicity in a 113-week oral study in mice at doses up to 40 mg/kg/day (10 times the maximum ...
-
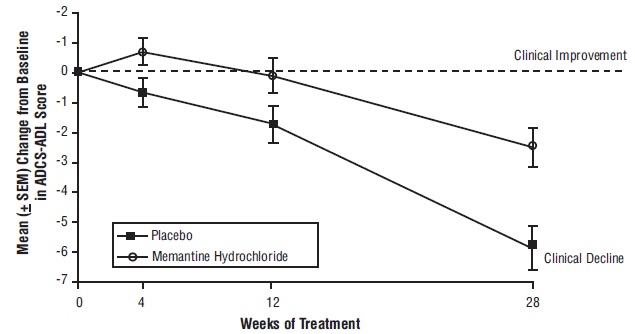
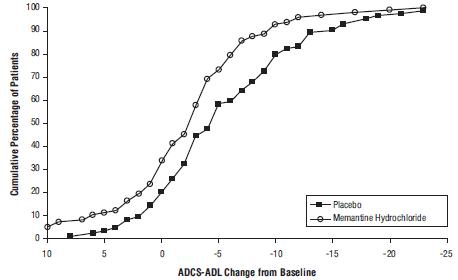
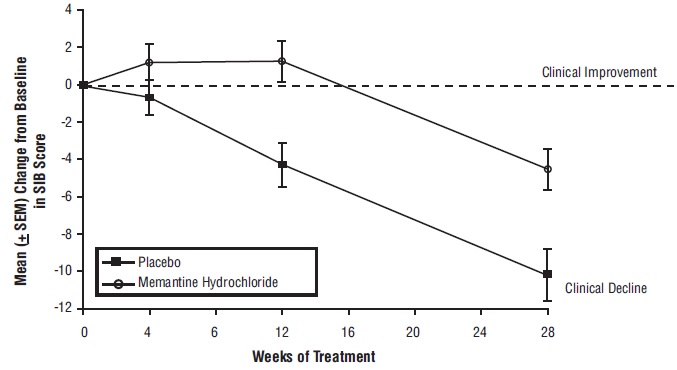
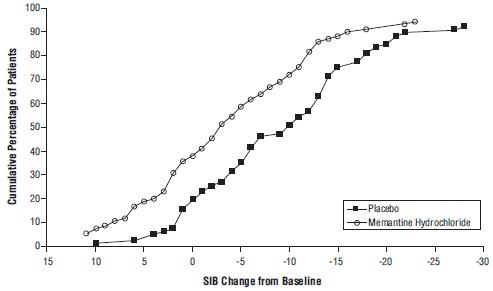
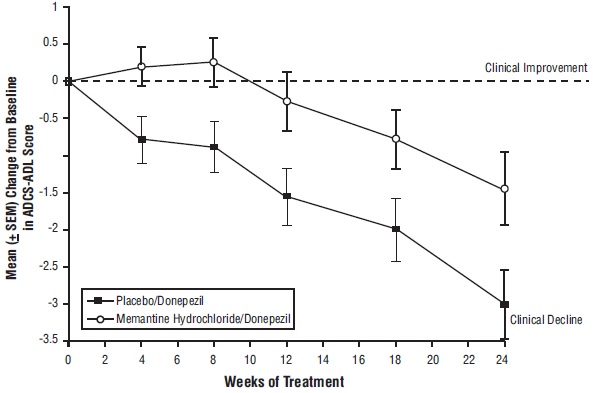
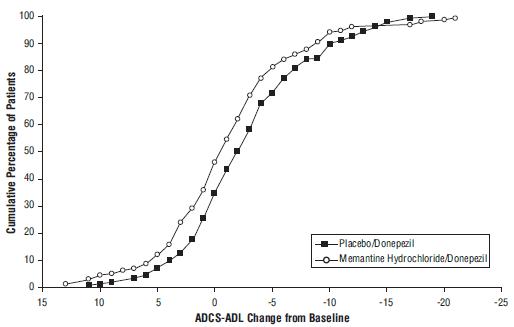
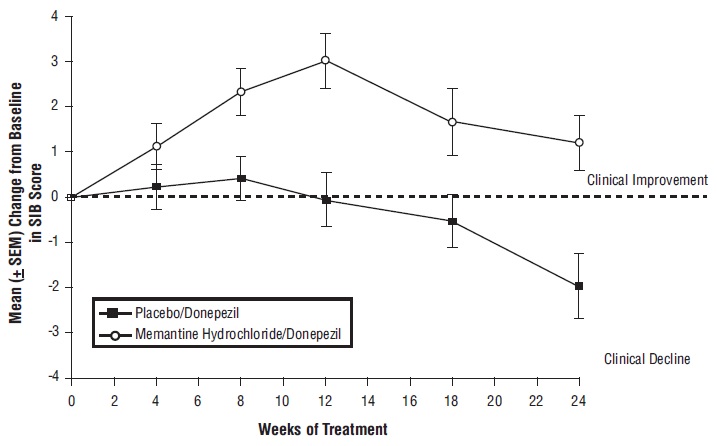
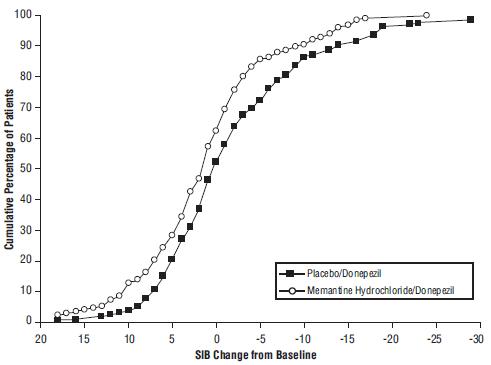
14 CLINICAL STUDIESThe effectiveness of memantine hydrochloride as a treatment for patients with moderate to severe Alzheimer’s disease was demonstrated in 2 randomized, double-blind, placebo-controlled clinical ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGMemantine Hydrochloride Tablets USP, 5 mg: white to off-white, modified caplet shaped, film-coated tablets debossed with ‘Z’ on one side and ‘01’ on other side - Bottles of ...
-
17 PATIENT COUNSELING INFORMATIONSee FDA-approved patient labeling (Patient Information). To assure safe and effective use of memantine hydrochloride, the following information and instructions provided in the patient ...
-
Patient InformationMemantine Hydrochloride Tablets USP [mem' an teen hye'' droe klor' ide] Read this Patient Information that comes with memantine hydrochloride tablets before you start taking them and each ...
-
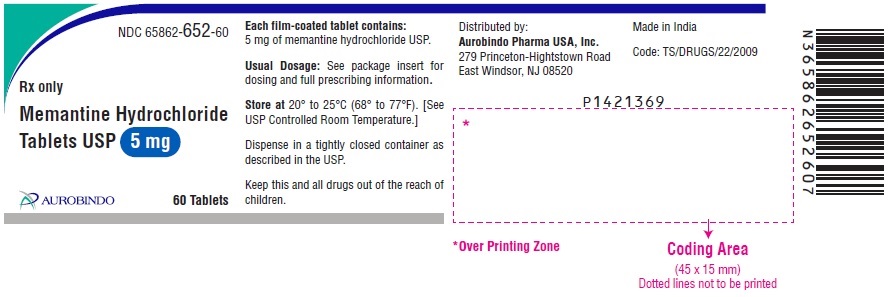
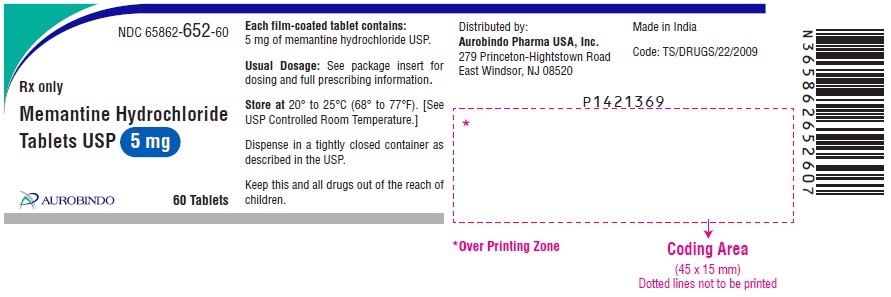
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 5 mg (60 Tablets Bottle)NDC 65862-652-60 - Rx only - Memantine Hydrochloride - Tablets USP 5 mg - AUROBINDO 60 Tablets
-
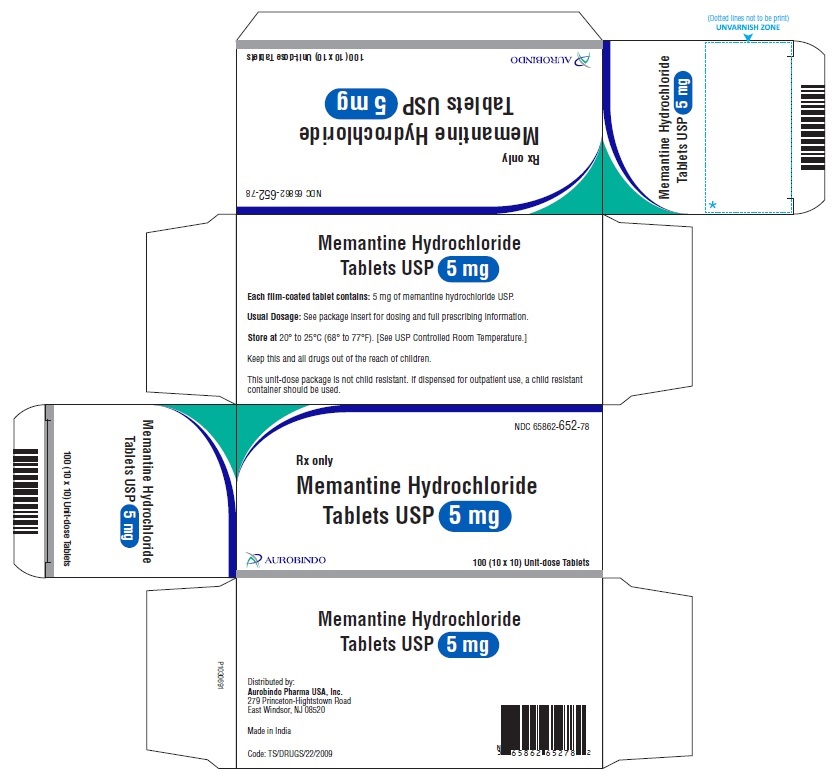
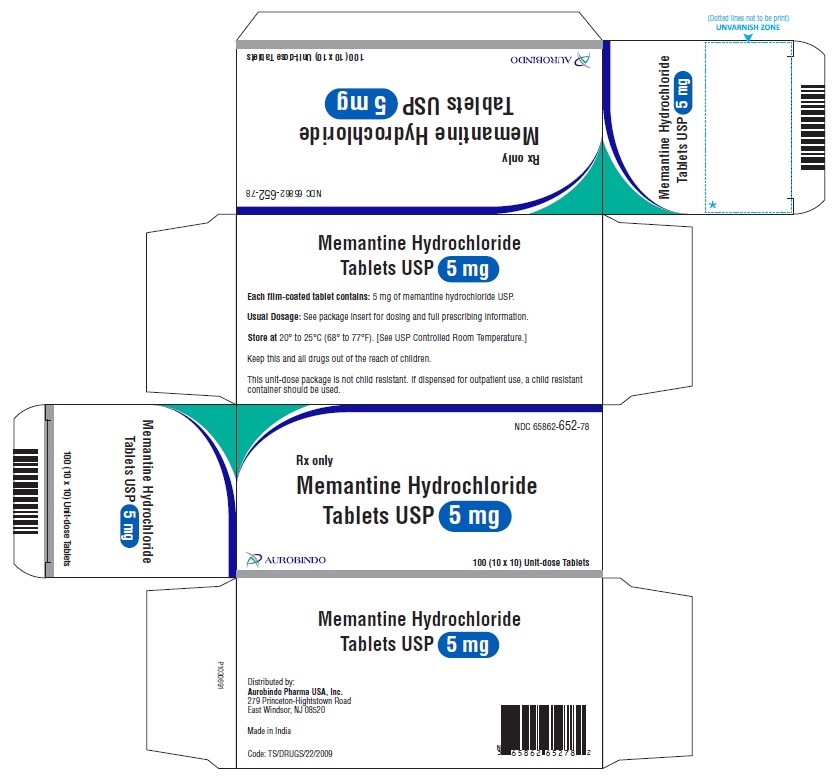
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 5 mg Blister Carton (10 x 10 Unit-dose)NDC-65862-652-78 - Rx only - Memantine Hydrochloride - Tablets USP 5 mg - AUROBINDO 100 (10 x 10) Unit-dose Tablets
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 10 mg (60 Tablets Bottle)NDC 65862-653-60 - Rx only - Memantine Hydrochloride - Tablets USP 10 mg - AUROBINDO 60 Tablets
-
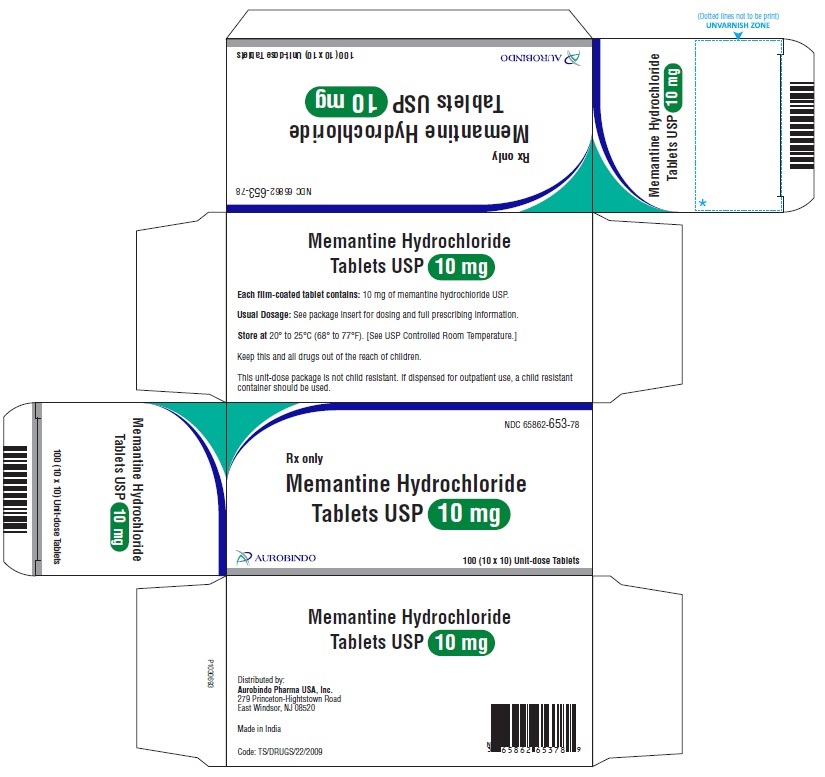
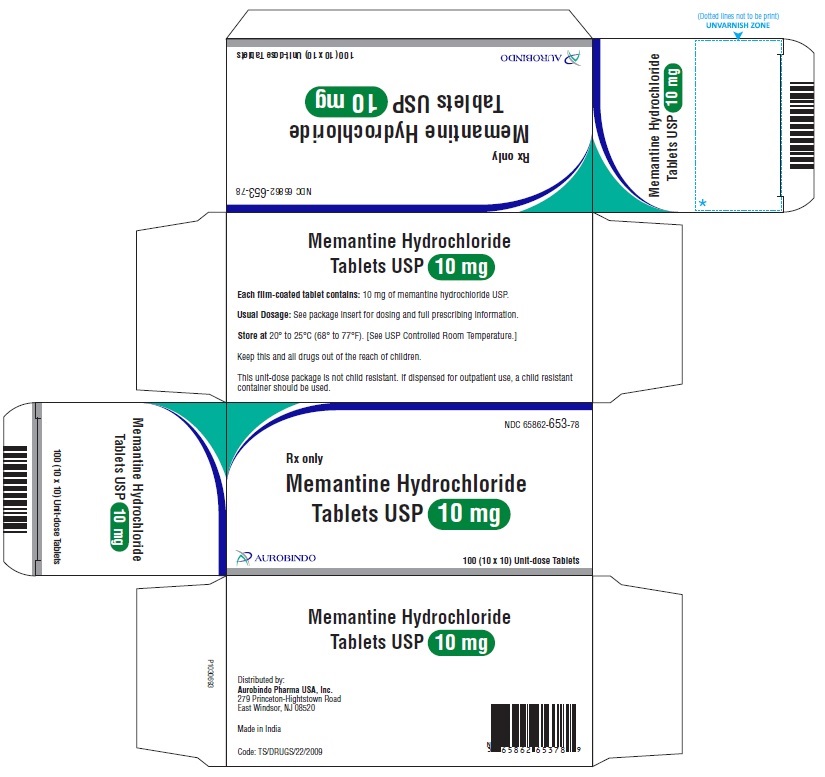
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 10 mg Blister Carton (10 x 10 Unit-dose)NDC-65862-653-78 - Rx only - Memantine Hydrochloride - Tablets USP 10 mg - AUROBINDO 100 (10 x 10) Unit-dose Tablets
-
INGREDIENTS AND APPEARANCEProduct Information