Label: VALSARTAN tablet, film coated
- NDC Code(s): 65862-570-10, 65862-570-30, 65862-570-99, 65862-571-10, view more
- Packager: Aurobindo Pharma Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 19, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use VALSARTAN TABLETS safely and effectively. See full prescribing information for VALSARTAN TABLETS. VALSARTAN tablets, for oral use ...
-
Table of ContentsTable of Contents
- BOXED WARNING (What is this?)
-
1 INDICATIONS AND USAGE1.1 Hypertension - Valsartan tablets are indicated for the treatment of hypertension, to lower blood pressure in adults and pediatric patients one year of age and older. Lowering blood pressure ...
-
2 DOSAGE AND ADMINISTRATION2.1 Important Dosage and Preparation Information - Valsartan tablets and oral suspension are not substitutable on a milligram-per-milligram basis. Do not combine two dosage forms to achieve the ...
-
3 DOSAGE FORMS AND STRENGTHS40 mg are yellow colored, ovaloid, beveled edge, biconvex, film-coated tablets debossed with 'I' on one side and '73' on other side with a score line separating 7 & 3. 80 mg are pale red ...
-
4 CONTRAINDICATIONSDo not use in patients with known hypersensitivity to any component. Do not coadminister aliskiren with valsartan tablets in patients with diabetes [see Drug Interactions (7.3)].
-
5 WARNINGS AND PRECAUTIONS5.1 Fetal Toxicity - Valsartan can cause fetal harm when administered to a pregnant woman. Use of drugs that act on the renin-angiotensin system during the second and third trimesters of ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly ...
-
7 DRUG INTERACTIONS7.1 Agents Increasing Serum Potassium - Concomitant use of valsartan with other agents that block the renin-angiotensin system, potassium-sparing diuretics (e.g., spironolactone, triamterene ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Valsartan can cause fetal harm when administered to a pregnant woman. Use of drugs that act on the renin-angiotensin system during the second and third ...
-
10 OVERDOSAGELimited data are available related to overdosage in humans. The most likely manifestations of overdosage would be hypotension and tachycardia; bradycardia could occur from parasympathetic (vagal ...
-
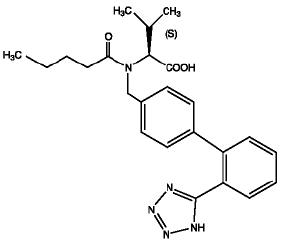
11 DESCRIPTIONValsartan is a nonpeptide, orally active, and specific angiotensin II receptor blocker acting on the AT1 receptor subtype. Valsartan is chemically described as ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Angiotensin II is formed from angiotensin I in a reaction catalyzed by angiotensin-converting enzyme (ACE, kininase II). Angiotensin II is the principal pressor agent ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - There was no evidence of carcinogenicity when valsartan was administered in the diet to mice and rats for up to 2 years at doses up ...
-
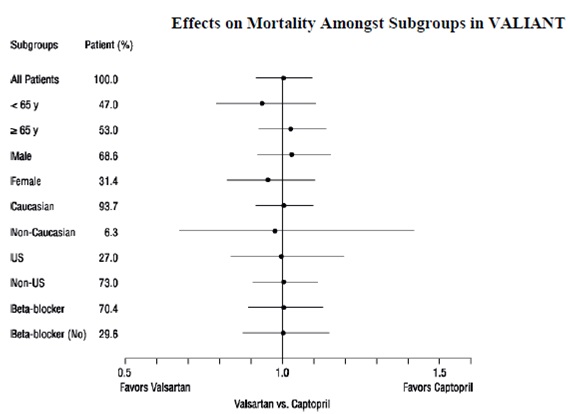
14 CLINICAL STUDIES14.1 Hypertension - Adult Hypertension - The antihypertensive effects of valsartan were demonstrated principally in 7 placebo-controlled, 4- to 12-week trials (1 in patients over 65 years ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGValsartan Tablets USP, 40 mg are yellow colored, ovaloid, beveled edge, biconvex, film-coated tablets debossed with 'I' on one side and '73' on other side with a score line separating 7 ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Pregnancy: Advise female patients of childbearing age about the consequences of exposure to valsartan during ...
-
PATIENT PACKAGE INSERTPATIENT INFORMATION - Valsartan (val sar' tan) Tablets USP - What is the most important information I should know about valsartan tablets? Valsartan tablets can cause ...
-
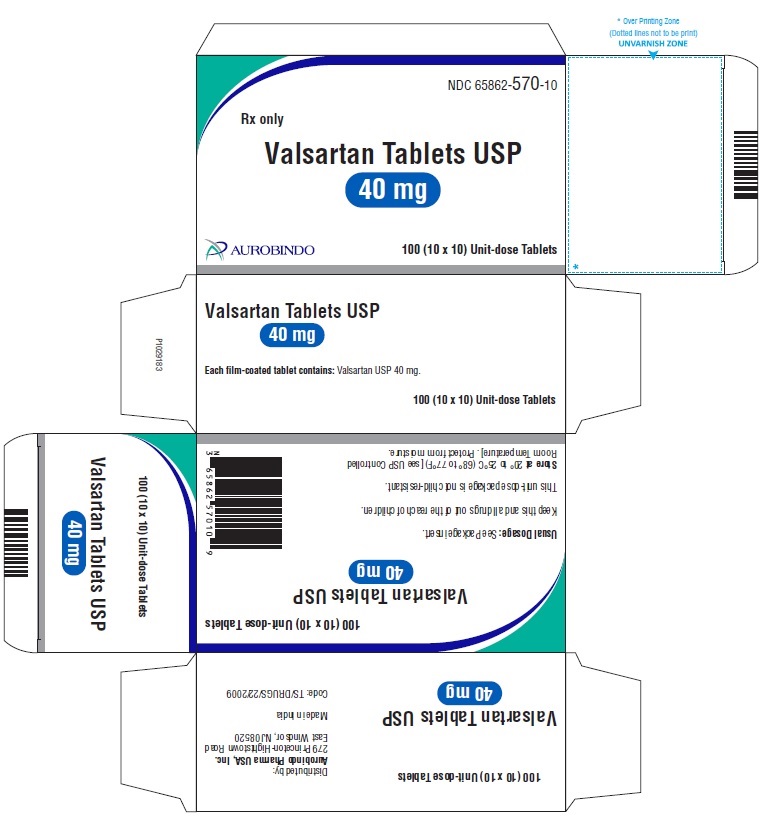
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 40 mg (30 Tablet Bottle)NDC 65862-570-30 Rx only - Valsartan Tablets USP - 40 mg - AUROBINDO 30 Tablets
-
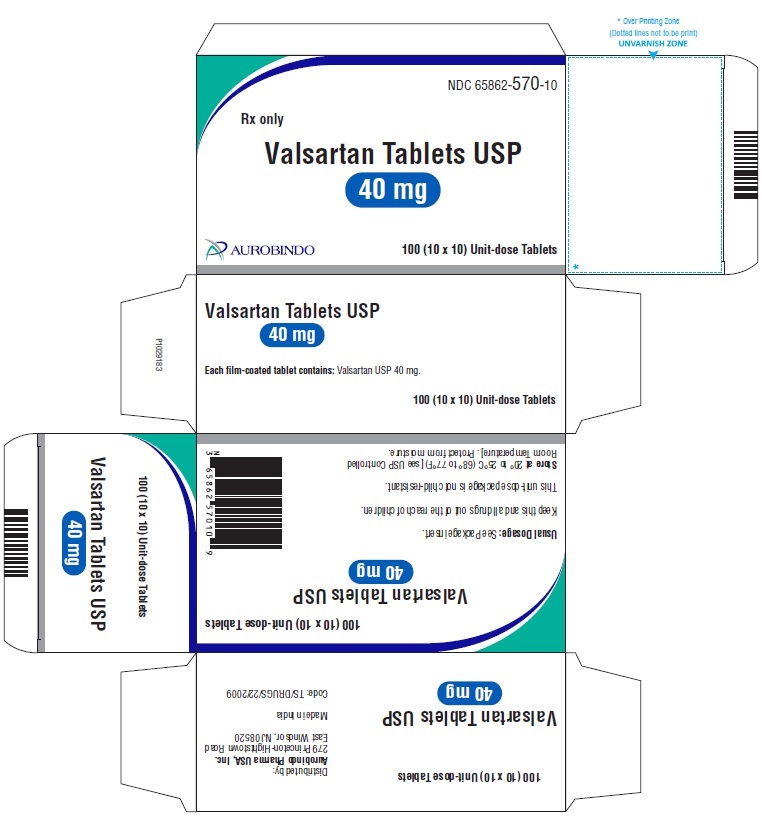
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 40 mg Blister Carton (10 x 10 Unit-dose)NDC 65862-570-10 - Rx only - Valsartan Tablets USP - 40 mg - AUROBINDO 100 (10 x 10) Unit-dose Tablets
-
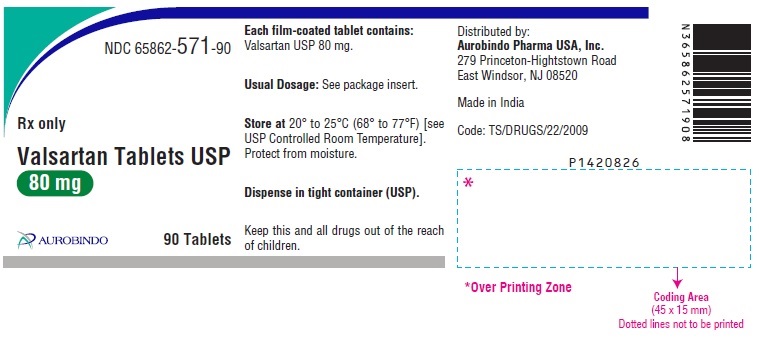
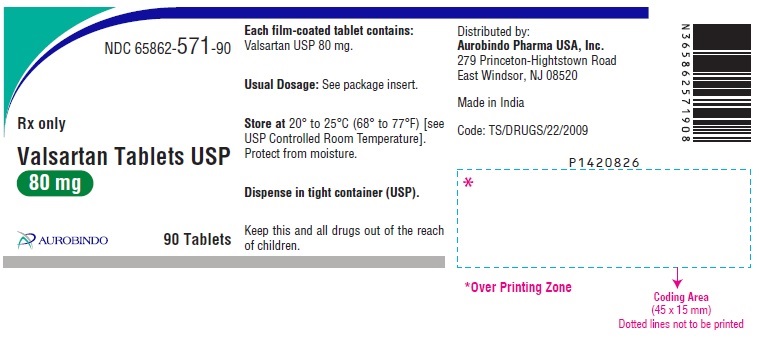
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 80 mg (90 Tablet Bottle)NDC 65862-571-90 - Rx only - Valsartan Tablets USP - 80 mg - AUROBINDO 90 Tablets
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 80 mg Blister Carton (10 x 10 Unit-dose)NDC 65862-571-10 - Rx only - Valsartan Tablets USP - 80 mg - AUROBINDO 100 (10 x 10) Unit-dose Tablets
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 160 mg (90 Tablet Bottle)NDC 65862-572-90 - Rx only - Valsartan Tablets USP - 160 mg - AUROBINDO 90 Tablets
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 160 mg Blister Carton (10 x 10 Unit-dose)NDC 65862-572-10 - Rx only - Valsartan Tablets USP - 160 mg - AUROBINDO 100 (10 x 10) Unit-dose Tablets
-
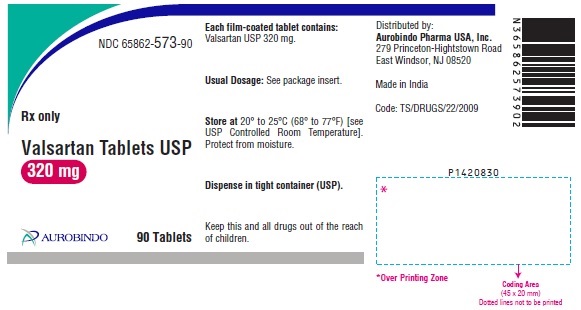
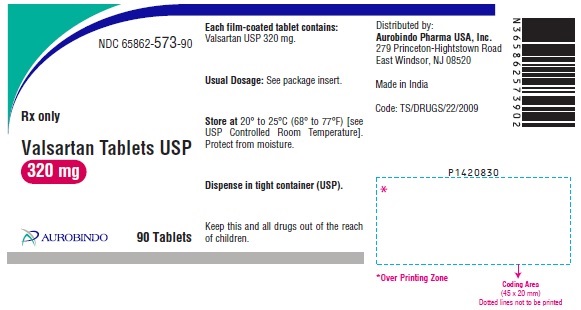
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 320 mg (90 Tablet Bottle)NDC 65862-573-90 - Rx only - Valsartan Tablets USP - 320 mg - AUROBINDO 90 Tablets
-
INGREDIENTS AND APPEARANCEProduct Information