Label: VALSARTAN AND HYDROCHLOROTHIAZIDE tablet, film coated
- NDC Code(s): 65862-547-10, 65862-547-90, 65862-547-99, 65862-548-10, view more
- Packager: Aurobindo Pharma Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use VALSARTAN AND HYDROCHLOROTHIAZIDE TABLETS safely and effectively. See full prescribing information for VALSARTAN AND ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: FETAL TOXICITY
- When pregnancy is detected, discontinue valsartan and hydrochlorothiazide as soon as possible [see Warnings and Precautions (5.1)].
- Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus [see Warnings and Precautions (5.1)].
-
1 INDICATIONS AND USAGEValsartan and hydrochlorothiazide tablets are indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular ...
-
2 DOSAGE AND ADMINISTRATION2.1 General Considerations - The usual starting dose is valsartan and hydrochlorothiazide tablets 160 mg/12.5 mg once daily. The dosage can be increased after 1 to 2 weeks of therapy to a ...
-
3 DOSAGE FORMS AND STRENGTHS80 mg/12.5 mg tablets are light orange colored, ovaloid, beveled edge, biconvex film-coated tablets debossed with ‘I’ on one side and ‘61’ on other side. 160 mg/12.5 mg tablets are dark red ...
-
4 CONTRAINDICATIONSValsartan and hydrochlorothiazide tablets are contraindicated in patients who are hypersensitive to any component of this product. Because of the hydrochlorothiazide component, this product is ...
-
5 WARNINGS AND PRECAUTIONS5.1 Fetal Toxicity - Valsartan and hydrochlorothiazide can cause fetal harm when administered to a pregnant woman. Use of drugs that act on the renin-angiotensin system during the second and ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical studies are conducted under widely varying conditions, adverse reactions rates observed in the clinical studies of a drug cannot be directly ...
-
7 DRUG INTERACTIONSValsartan and Hydrochlorothiazide: Lithium: Increases in serum lithium concentrations and lithium toxicity have been reported during concomitant administration of lithium with angiotensin II ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Valsartan and hydrochlorothiazide can cause fetal harm when administered to a pregnant woman. Use of drugs that act on the renin-angiotensin system during the ...
-
10 OVERDOSAGELimited data are available related to overdosage in humans. The most likely manifestations of overdosage would be hypotension and tachycardia; bradycardia could occur from parasympathetic (vagal ...
-
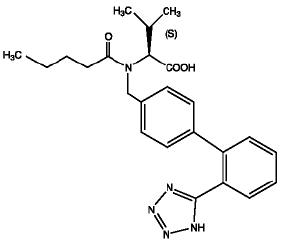
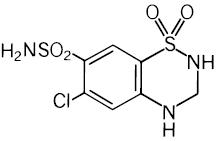
11 DESCRIPTIONValsartan and hydrochlorothiazide is a combination of valsartan, an orally active, specific angiotensin II receptor blocker (ARB) acting on the AT1 receptor subtype, and hydrochlorothiazide, a ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Angiotensin II is formed from angiotensin I in a reaction catalyzed by angiotensin-converting enzyme (ACE, kininase II). Angiotensin II is the principal pressor agent ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Valsartan and Hydrochlorothiazide: No carcinogenicity, mutagenicity, or fertility studies have been conducted with the combination of ...
-
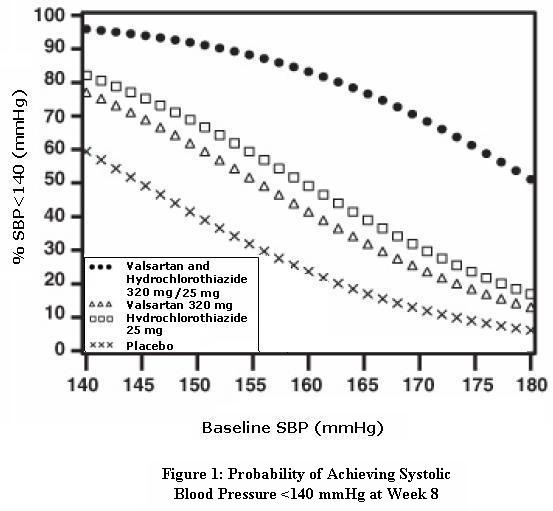
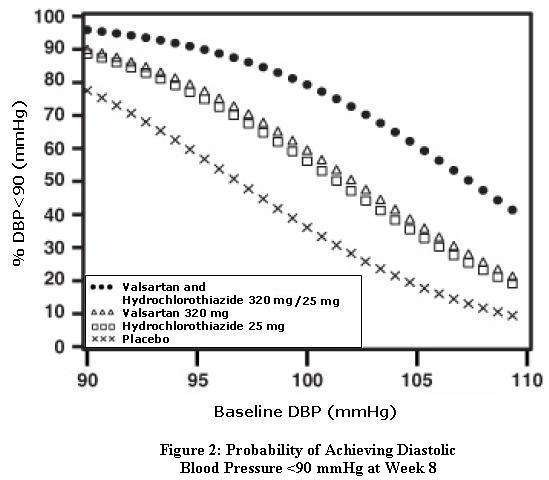
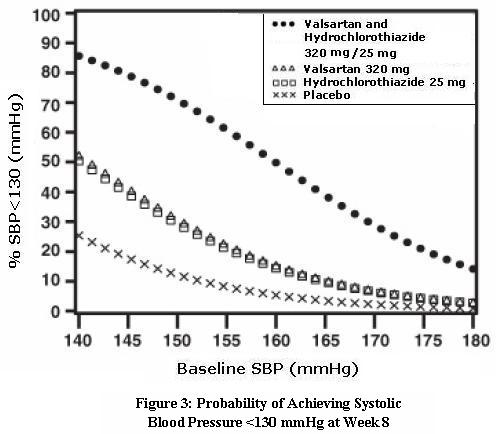
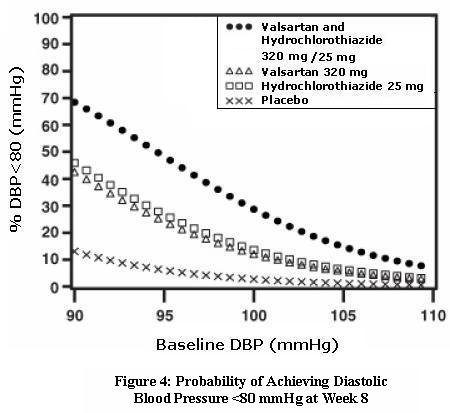
14 CLINICAL STUDIES14.1 Hypertension - Valsartan and Hydrochlorothiazide: In controlled clinical trials including over 7600 patients, 4372 patients were exposed to valsartan (80, 160, and 320 mg) and concomitant ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGValsartan and hydrochlorothiazide tablets, USP are available as non-scored tablets containing valsartan and hydrochlorothiazide 80 mg/12.5 mg, 160 mg/12.5 mg, 160 mg/25 mg, 320 mg/12.5 mg, and ...
-
17 PATIENT COUNSELING INFORMATIONInformation for Patients Advise the patient to read the FDA-approved patient labeling (Patient Information). Pregnancy: Advise female patients of childbearing age about the consequences of ...
-
FDA-Approved Patient LabelingPATIENT INFORMATION - Valsartan and Hydrochlorothiazide Tablets, USP - (val sar' tan and hye'' droe klor'' oh thye' a zide) Read the Patient Information that comes with valsartan and ...
-
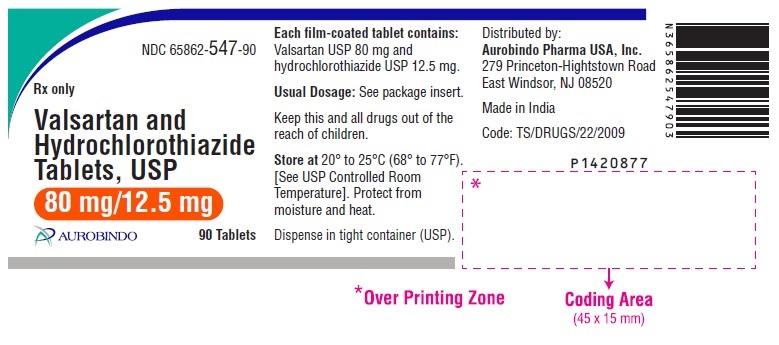
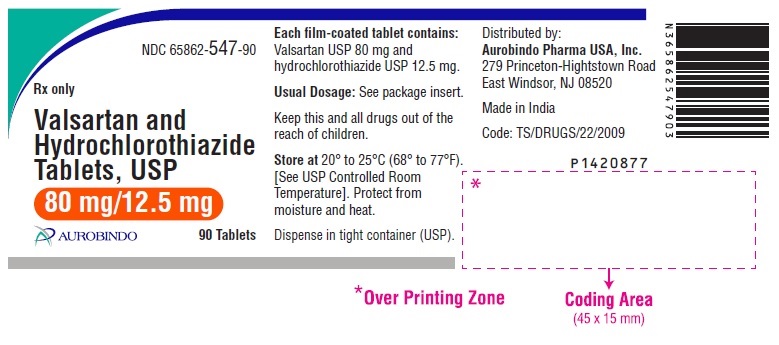
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 80 mg/12.5 mg (90 Tablets Bottle)NDC 65862-547-90 - Rx only - Valsartan and - Hydrochlorothiazide - Tablets, USP - 80 mg/12.5 mg - AUROBINDO 90 Tablets
-
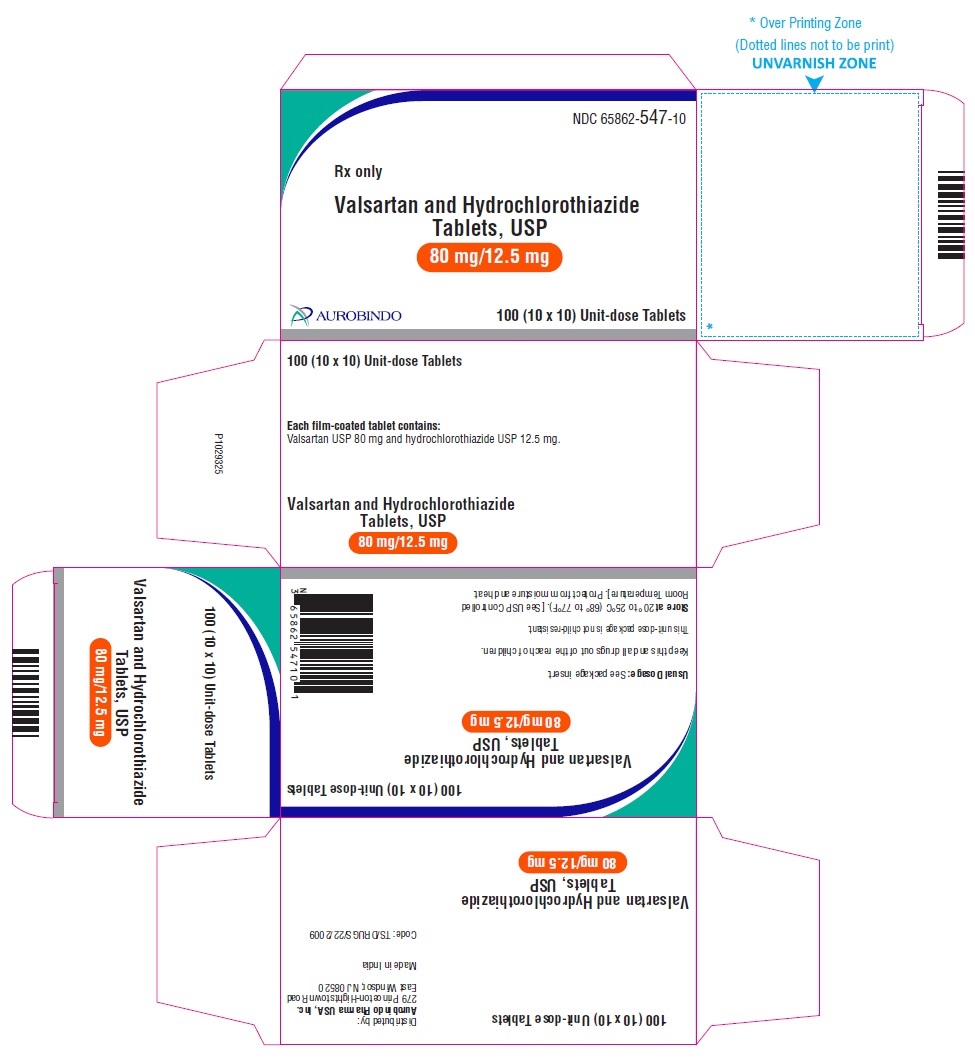
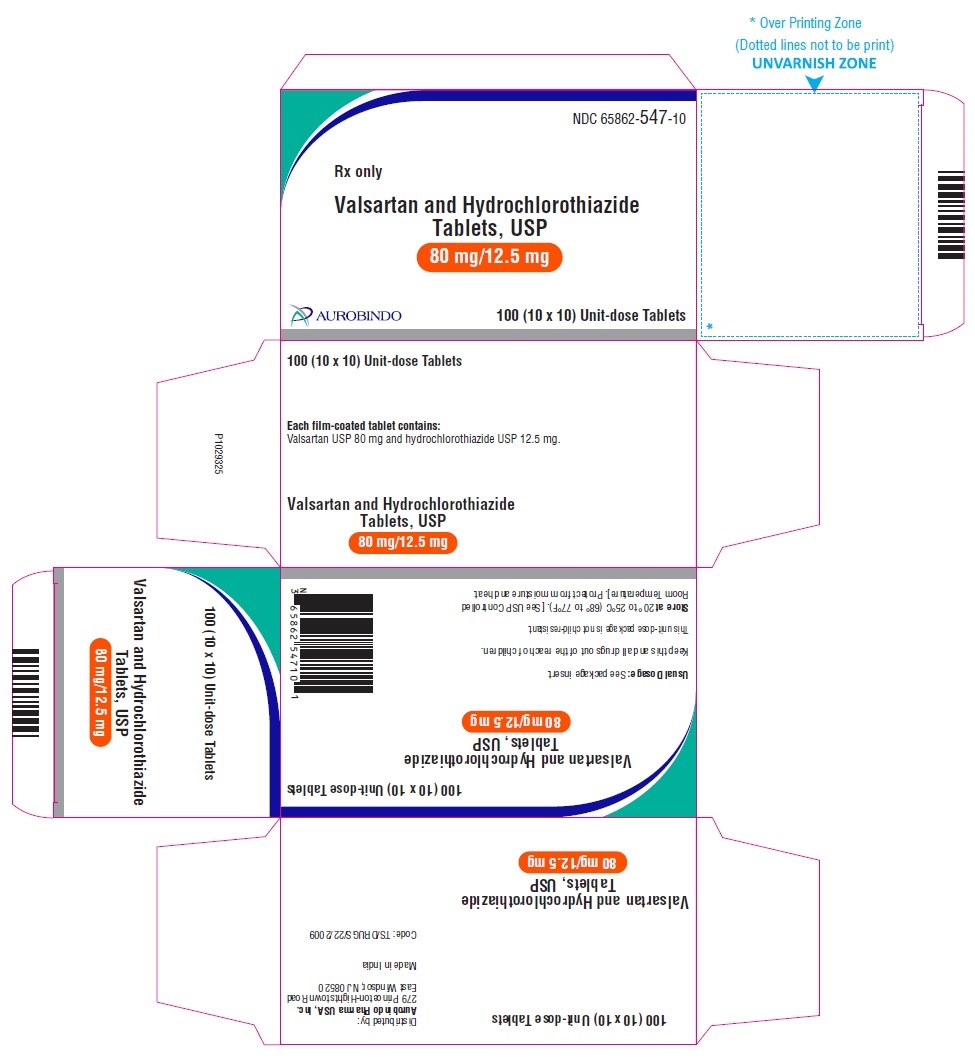
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 80 mg/12.5 mg Blister Carton (10 x 10 Unit-dose)NDC 65862-547-10 - Rx only - Valsartan and Hydrochlorothiazide Tablets, USP - 80 mg/12.5 mg - AUROBINDO 100 (10 x 10) Unit-dose Tablets
-
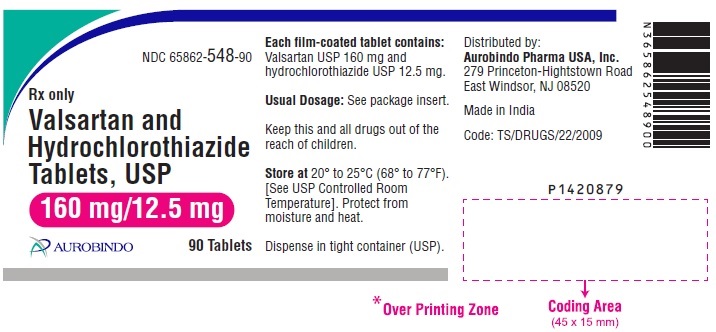
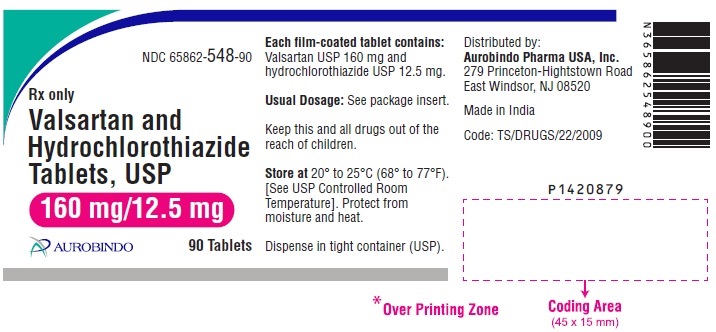
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 160 mg/12.5 mg (90 Tablets Bottle)NDC 65862-548-90 - Rx only - Valsartan and - Hydrochlorothiazide - Tablets, USP - 160 mg/12.5 mg - AUROBINDO 90 Tablets
-
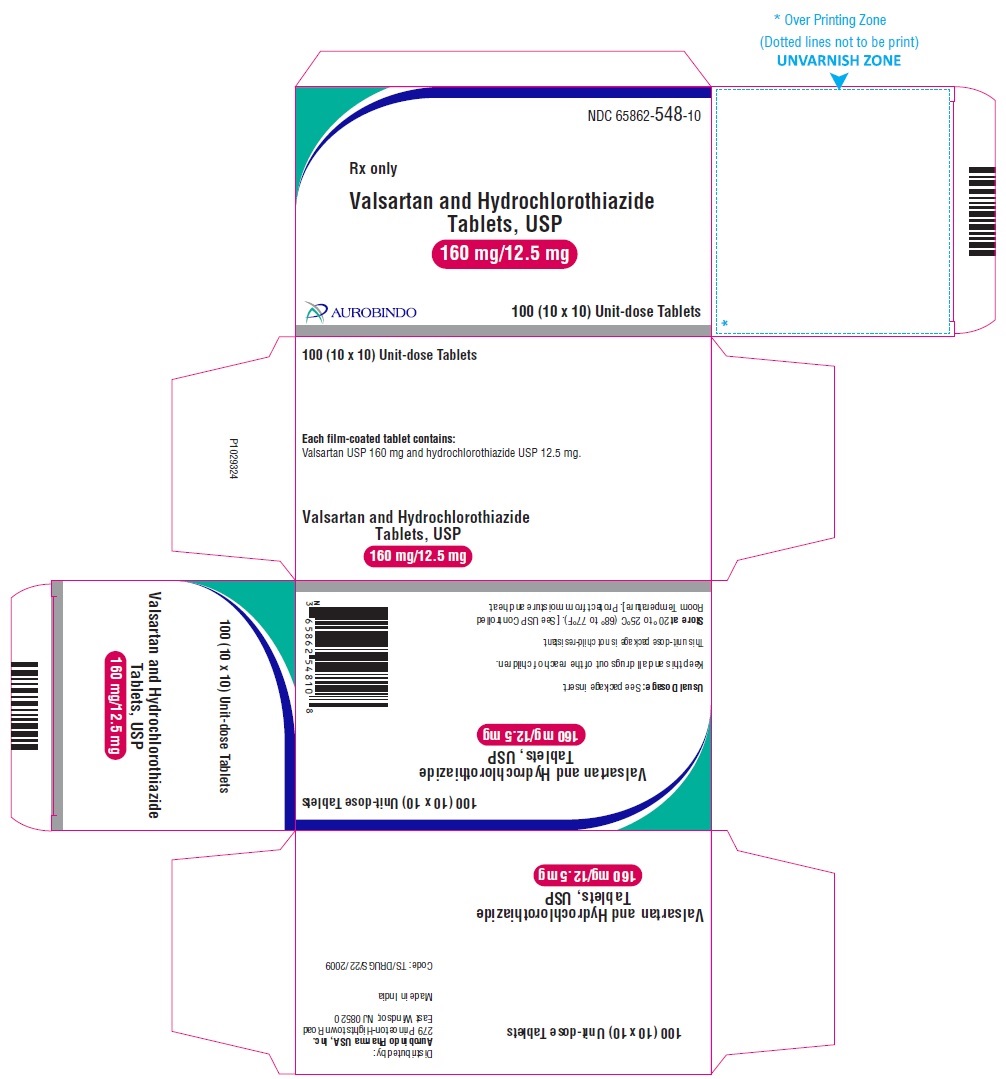
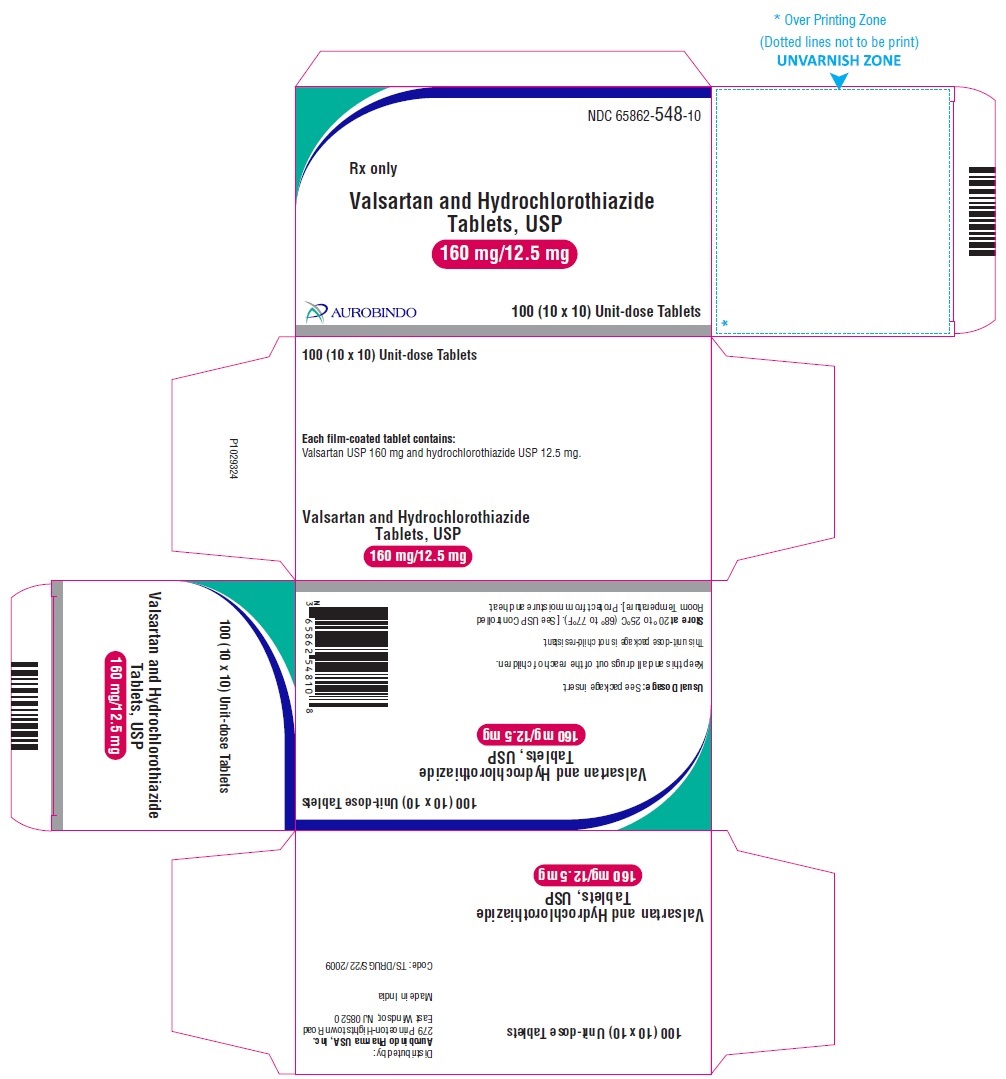
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 160 mg/12.5 mg Blister Carton (10 x 10 Unit-dose)NDC 65862-548-10 - Rx only - Valsartan and Hydrochlorothiazide Tablets, USP - 160 mg/12.5 mg - AUROBINDO 100 (10 x 10) Unit-dose Tablets
-
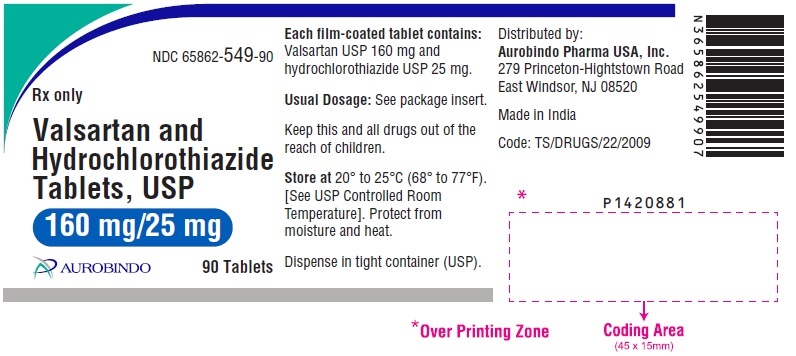
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 160 mg/25 mg (90 Tablets Bottle)NDC 65862-549-90 - Rx only - Valsartan and - Hydrochlorothiazide - Tablets, USP - 160 mg/25 mg - AUROBINDO 90 Tablets
-
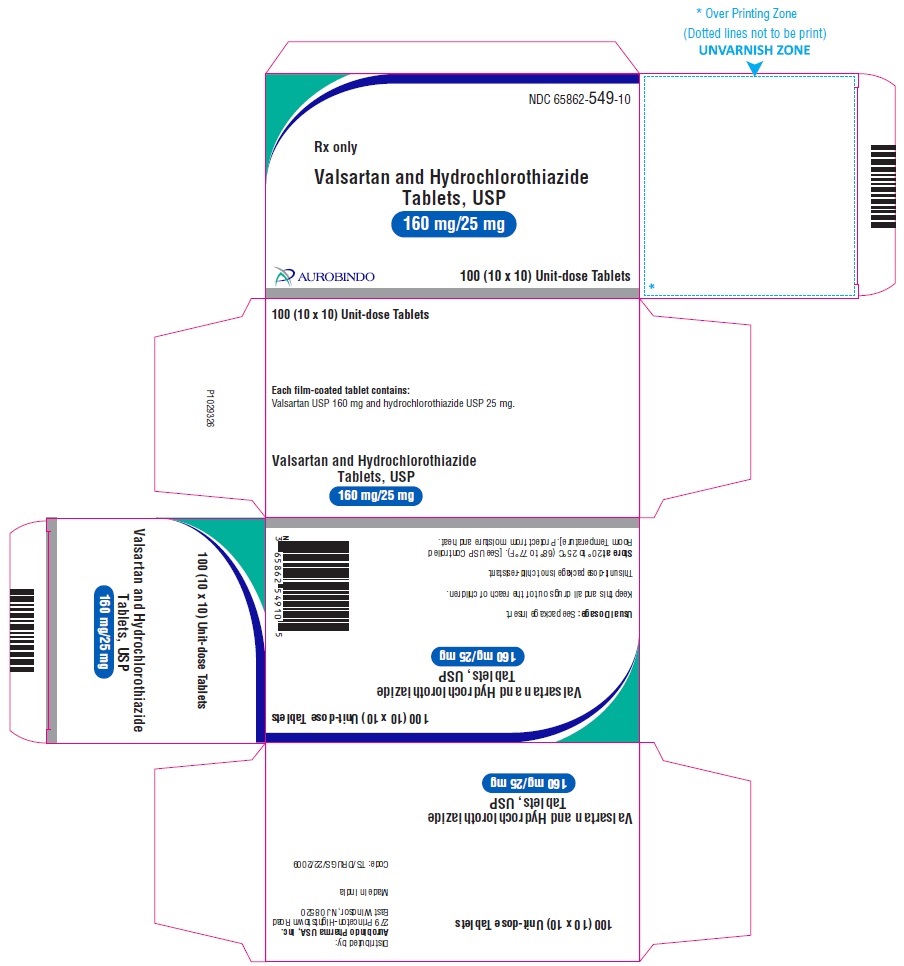
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 160 mg/25 mg Blister Carton (10 x 10 Unit-dose)NDC 65862-549-10 - Rx only - Valsartan and Hydrochlorothiazide Tablets, USP - 160 mg/25 mg - AUROBINDO 100 (10 x 10) Unit-dose Tablets
-
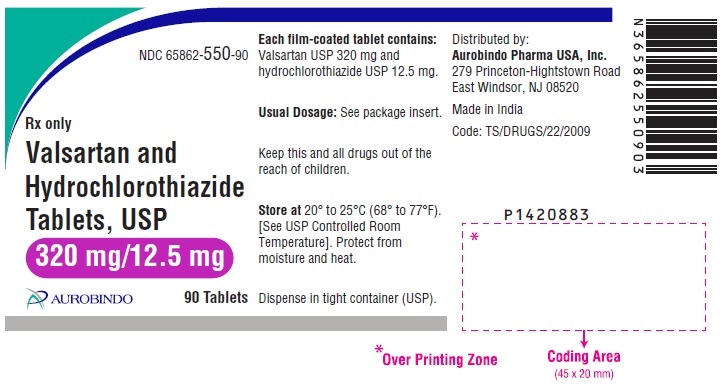
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 320 mg/12.5 mg (90 Tablets Bottle)NDC 65862-550-90 - Rx only Valsartan and - Hydrochlorothiazide - Tablets, USP - 320 mg/12.5 mg - AUROBINDO 90 Tablets
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 320 mg/25 mg (90 Tablets Bottle)NDC 65862-551-90 - Rx only - Valsartan and - Hydrochlorothiazide - Tablets, USP - 320 mg/25 mg - AUROBINDO 90 Tablets
-
INGREDIENTS AND APPEARANCEProduct Information