Label: CEFPROZIL powder, for suspension
- NDC Code(s): 65862-099-01, 65862-099-50, 65862-099-75, 65862-100-01, view more
- Packager: Aurobindo Pharma Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONTo reduce the development of drug-resistant bacteria and maintain the effectiveness of cefprozil and other antibacterial drugs, cefprozil should be used only to treat or prevent infections that ...
-
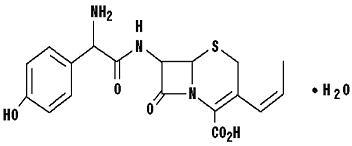
DESCRIPTIONCefprozil is a semi-synthetic broad-spectrum cephalosporin antibiotic. Cefprozil is a cis and trans isomeric mixture (≥90% cis). The chemical name for the monohydrate is ...
-
CLINICAL PHARMACOLOGYThe pharmacokinetic data were derived from the capsule formulation; however, bioequivalence has been demonstrated for the oral solution, capsule, tablet, and suspension formulations under ...
-
INDICATIONS AND USAGETo reduce the development of drug-resistant bacteria and maintain the effectiveness of cefprozil and other antibacterial drugs, cefprozil should be used only to treat or prevent infections that ...
-
CONTRAINDICATIONSCefprozil for oral suspension is contraindicated in patients with known allergy to the cephalosporin class of antibiotics.
-
WARNINGSBEFORE THERAPY WITH CEFPROZIL IS INSTITUTED, CAREFUL INQUIRY SHOULD BE MADE TO DETERMINE WHETHER THE PATIENT HAS HAD PREVIOUS HYPERSENSITIVITY REACTIONS TO CEFPROZIL, CEPHALOSPORINS ...
-
PRECAUTIONSGeneral - Prescribing cefprozil in the absence of proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the ...
-
ADVERSE REACTIONSThe adverse reactions to cefprozil are similar to those observed with other orally administered cephalosporins. Cefprozil was usually well tolerated in controlled clinical trials. Approximately ...
-
OVERDOSAGESingle 5000 mg/kg oral doses of cefprozil caused no mortality or signs of toxicity in adult, weanling, or neonatal rats, or adult mice. A single oral dose of 3000 mg/kg caused diarrhea and loss ...
-
DOSAGE AND ADMINISTRATIONCefprozil for oral suspension is administered orally. Population/Infection Dosage - (mg) Duration (days) a In the treatment of infections due to ...
-
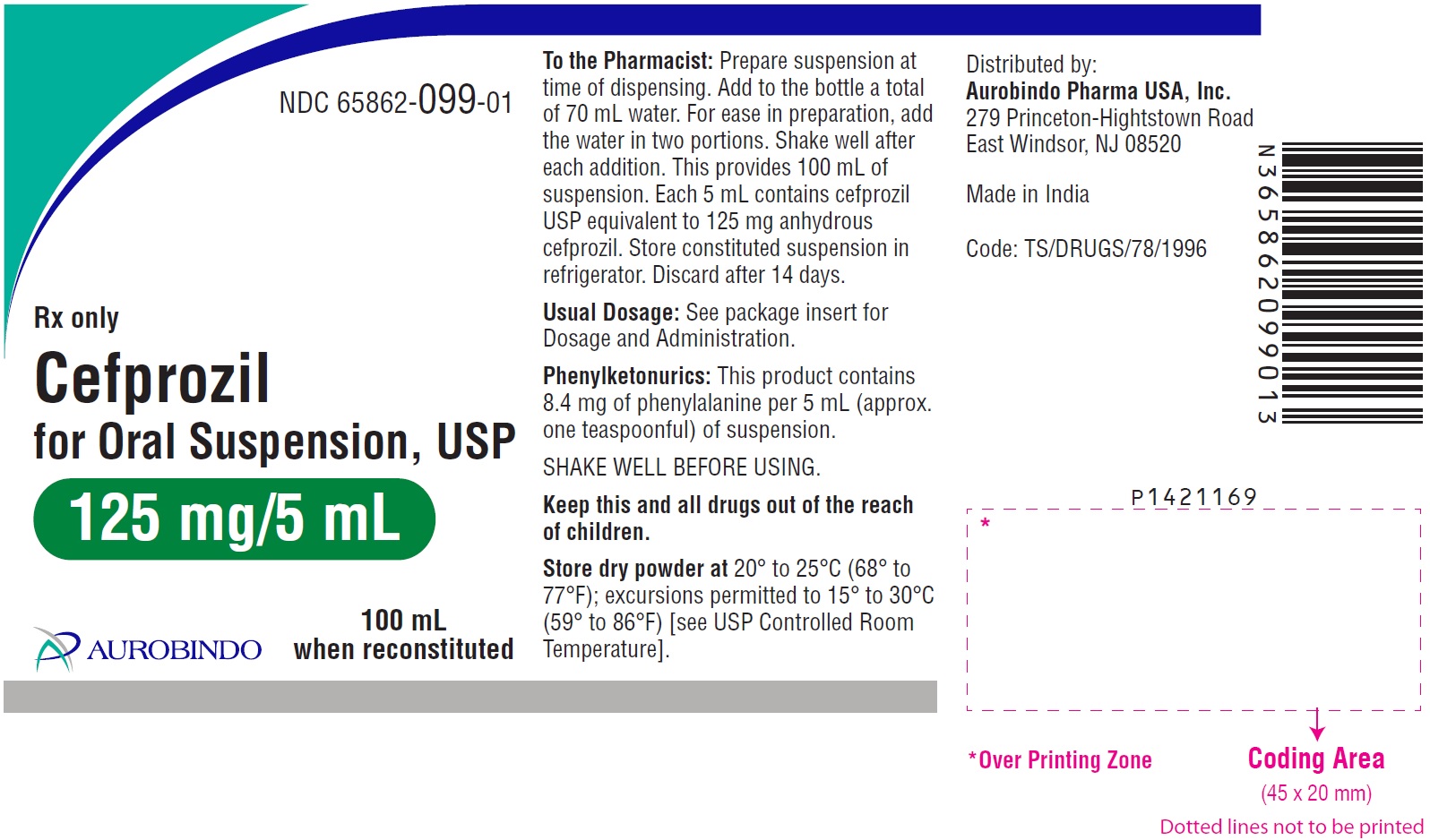
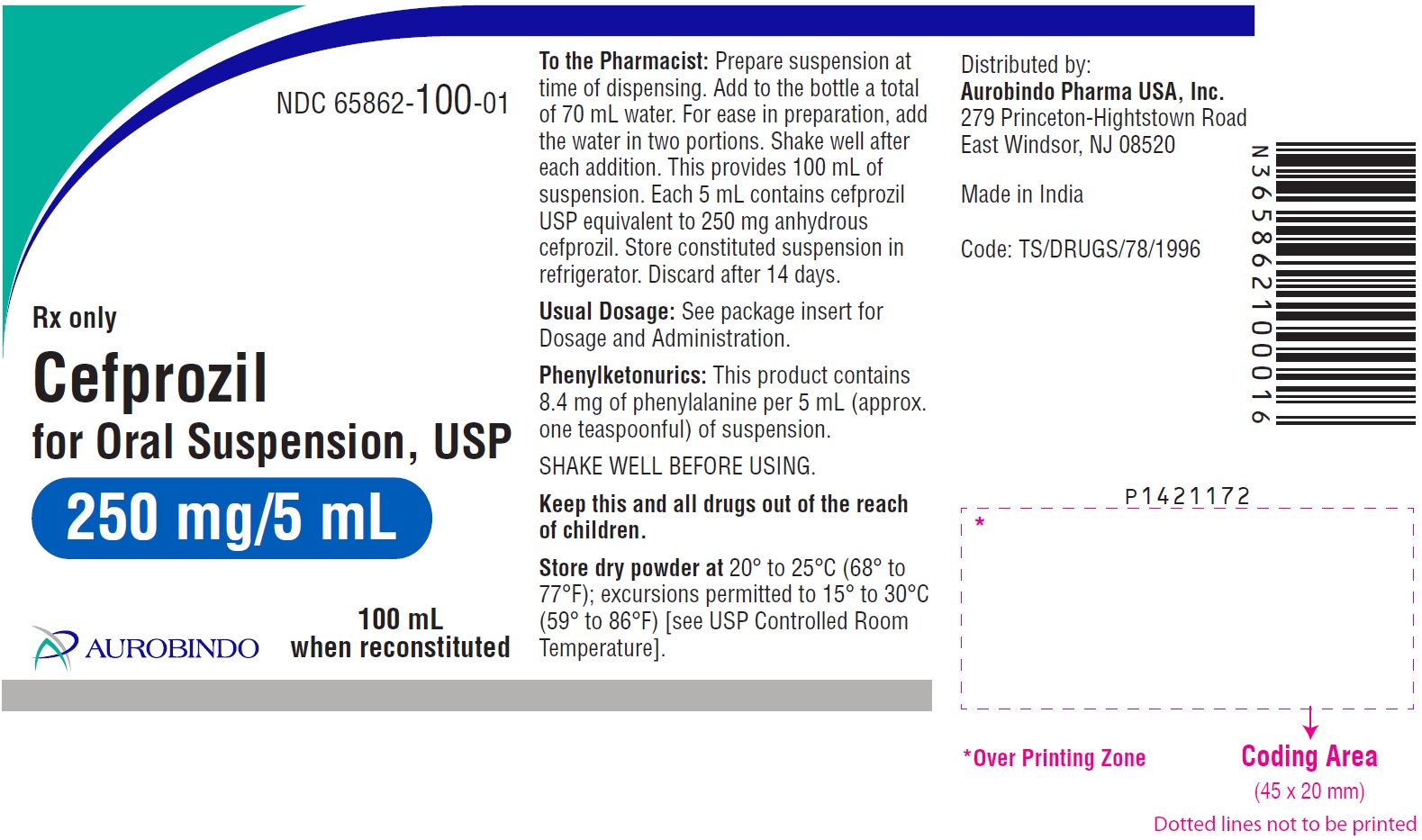
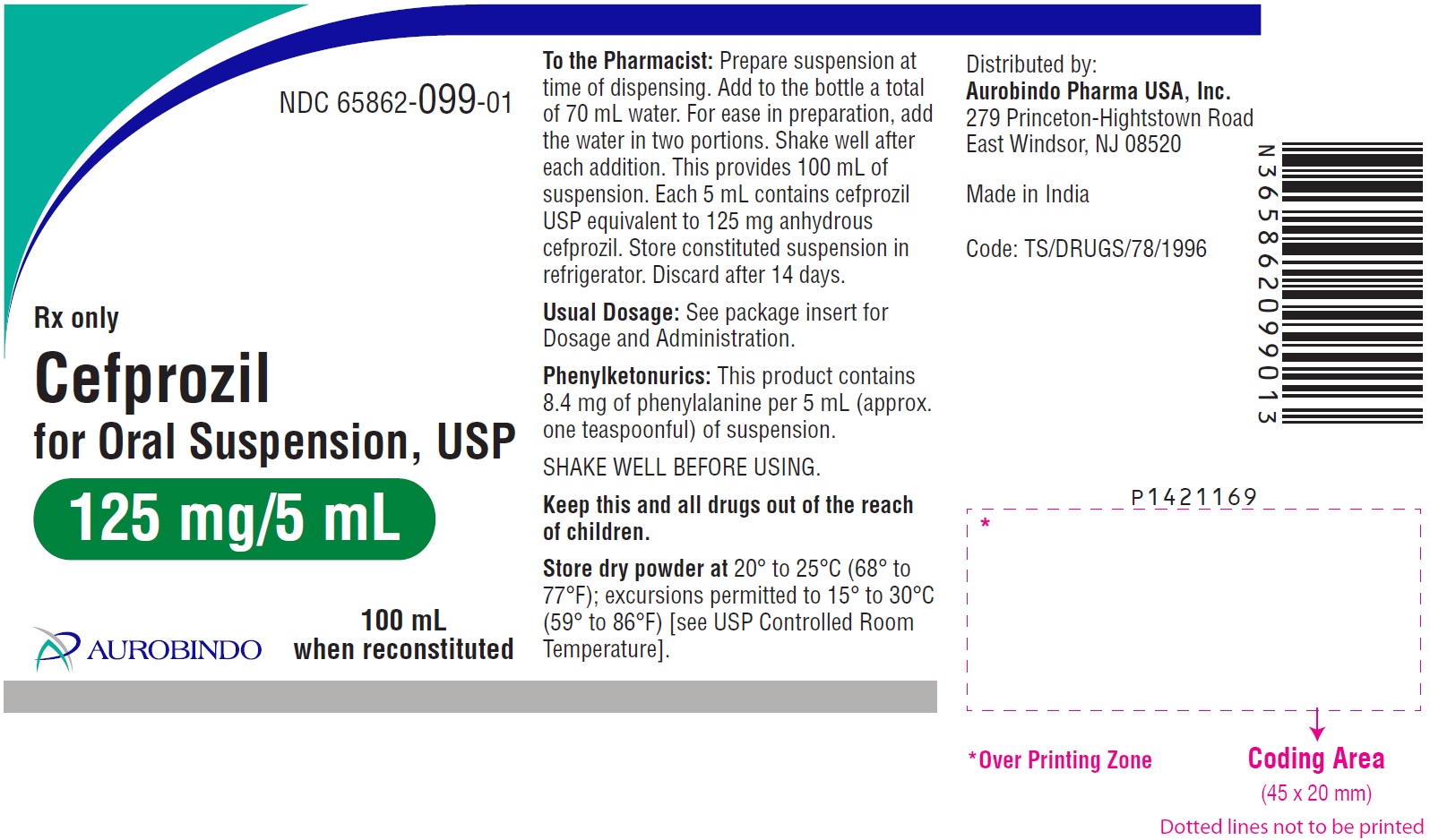
HOW SUPPLIEDCefprozil for Oral Suspension, USP 125 mg/5 mL: Each 5 mL of reconstituted suspension contains cefprozil USP equivalent to anhydrous cefprozil 125 mg. 50 mL ...
-
CLINICAL STUDIESStudy One: In a controlled clinical study of acute otitis media performed in the United States where significant rates of β-lactamase-producing organisms were found, cefprozil was compared to ...
-
Distributed by: Aurobindo Pharma USA, Inc. 279 Princeton-Hightstown Road - East Windsor, NJ 08520 - Manufactured by: Aurobindo Pharma Limited - Hyderabad-500 032, India - Revised: 12/2021
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 125 mg/5 mL (100 mL Bottle)NDC 65862-099-01 - Rx only - Cefprozil - for Oral Suspension, USP - 125 mg/5 mL - 100 mL - when reconstituted - AUROBINDO
-
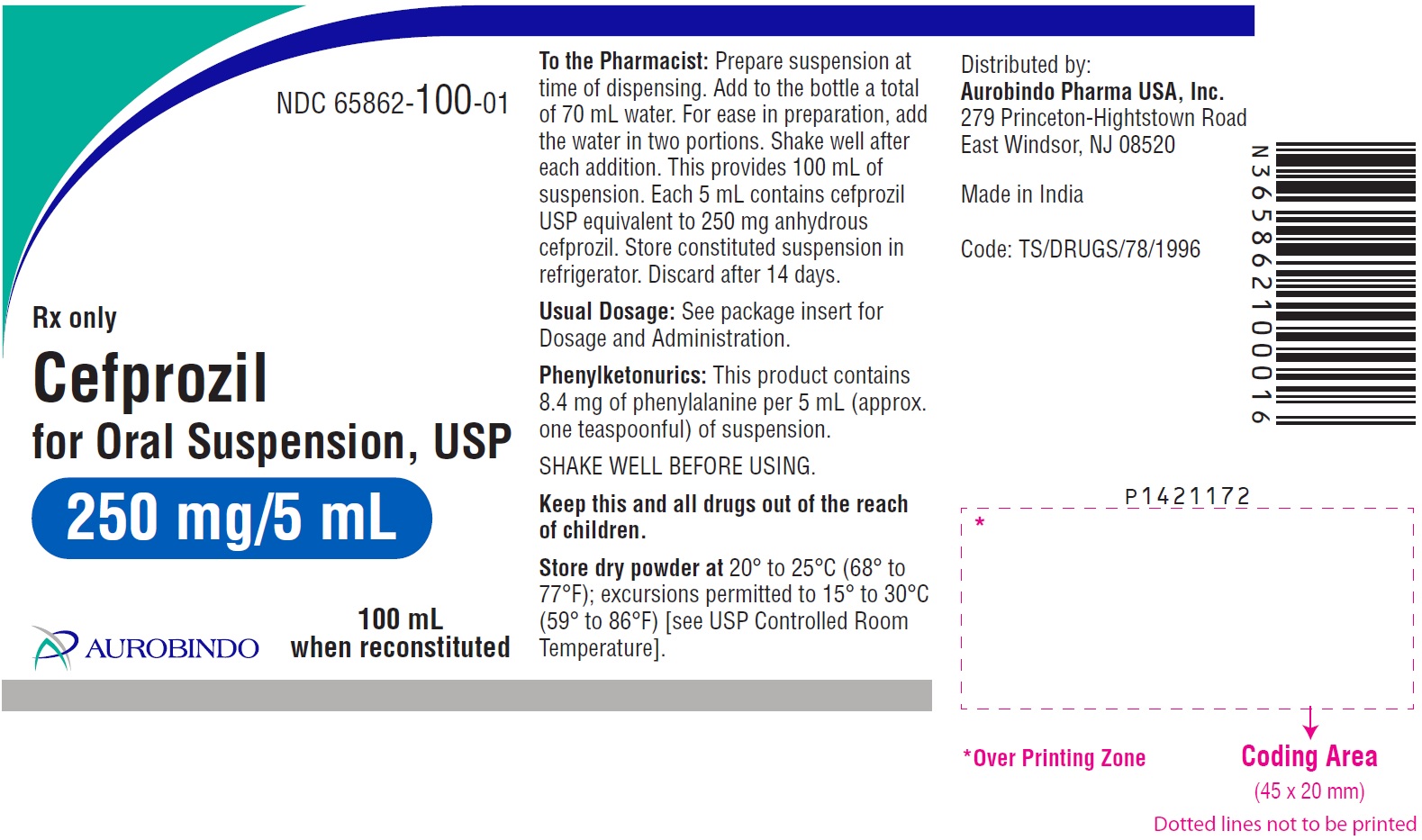
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 250 mg/5 mL (100 mL Bottle)NDC 65862-100-01 - Rx only - Cefprozil - for Oral Suspension, USP - 250 mg/5 mL - 100 mL - when reconstituted - AUROBINDO
-
INGREDIENTS AND APPEARANCEProduct Information