Label: CEFADROXIL powder, for suspension
- NDC Code(s): 65862-083-01, 65862-083-50, 65862-084-01, 65862-084-50, view more
- Packager: Aurobindo Pharma Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 9, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONTo reduce the development of drug-resistant bacteria and maintain the effectiveness of cefadroxil and other antibacterial drugs, cefadroxil should be used only to treat or prevent infections that ...
-
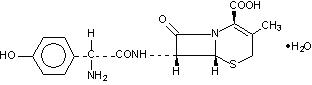
DESCRIPTIONCefadroxil monohydrate, USP is a semisynthetic cephalosporin antibiotic intended for oral administration. It is a white to yellowish-white crystalline powder. It is soluble in water and it is ...
-
CLINICAL PHARMACOLOGYCefadroxil is rapidly absorbed after oral administration. Following single doses of 500 mg and 1000 mg, average peak serum concentrations were approximately 16 and 28 mcg/mL, respectively ...
-
INDICATIONS AND USAGECefadroxil is indicated for the treatment of patients with infection caused by susceptible strains of the designated organisms in the following diseases: Urinary tract infections caused by E ...
-
CONTRAINDICATIONSCefadroxil is contraindicated in patients with known allergy to the cephalosporin group of antibiotics.
-
WARNINGSBEFORE THERAPY WITH CEFADROXIL IS INSTITUTED, CAREFUL INQUIRY SHOULD BE MADE TO DETERMINE WHETHER THE PATIENT HAS HAD PREVIOUS HYPERSENSITIVITY REACTIONS TO CEFADROXIL, CEPHALOSPORINS ...
-
PRECAUTIONSGeneral - Cefadroxil should be used with caution in the presence of markedly impaired renal function (creatinine clearance rate of less than 50 mL/min/1.73 m2). (See DOSAGE AND ...
-
ADVERSE REACTIONSGastrointestinal - Onset of pseudomembranous colitis symptoms may occur during or after antibiotic treatment (see WARNINGS). Dyspepsia, nausea and vomiting have been reported rarely. Diarrhea ...
-
OVERDOSAGEA study of children under six years of age suggested that ingestion of less than 250 mg/kg of cephalosporins is not associated with significant outcomes. No action is required other than ...
-
DOSAGE AND ADMINISTRATIONCefadroxil is acid-stable and may be administered orally without regard to meals. Administration with food may be helpful in diminishing potential gastrointestinal complaints occasionally ...
-
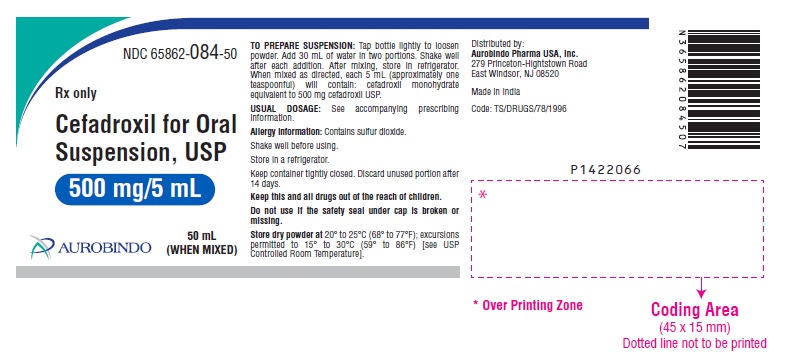
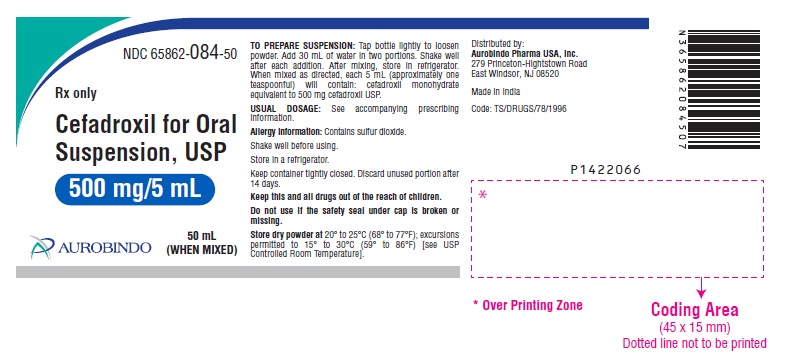
HOW SUPPLIEDCefadroxil for Oral Suspension, USP is an off white to light orange colored granular powder which after reconstitution is orange colored and is orange-pineapple flavored, and is supplied as ...
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 250 mg/5 mL (50 mL WHEN MIXED)NDC 65862-083-50 - Rx only - Cefadroxil for Oral - Suspension, USP - 250 mg/5 mL - 50 mL - AUROBINDO (WHEN MIXED)
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 500 mg/5 mL (50 mL WHEN MIXED)NDC 65862-084-50 - Rx only - Cefadroxil for Oral - Suspension, USP - 500 mg/5 mL - 50 mL - AUROBINDO (WHEN MIXED)
-
INGREDIENTS AND APPEARANCEProduct Information