Label: LIDOCAINE ointment
- NDC Code(s): 65162-918-30, 65162-918-38, 65162-918-53
- Packager: Amneal Pharmaceuticals LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 28, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Lidocaine Ointment USP, 5% contains a local anesthetic agent and is administered topically. See INDICATIONS AND USAGE for specific uses.
Lidocaine Ointment USP, 5% contains lidocaine USP, which is chemically designated as acetamide, 2-(diethylamino)-N-(2,6-dimethylphenyl)-,and has the following structural formula:
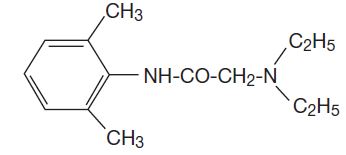
Composition of Lidocaine Ointment USP, 5%: acetamide, 2-(diethylamino)-N-(2,6-dimethylphenyl)-, (lidocaine) 5% in a water miscible ointment vehicle containing polyethylene glycols.
-
CLINICAL PHARMACOLOGY
Mechanism of action
Lidocaine stabilizes the neuronal membrane by inhibiting the ionic fluxes required for the initiation and conduction of impulses, thereby effecting local anesthetic action.
Onset of anesthesia
Lidocaine Ointment 5% effects local, topical anesthesia. The onset of action is 3 to 5 minutes. It is ineffective when applied to intact skin.
Hemodynamics
Excessive blood levels may cause changes in cardiac output, total peripheral resistance, and mean arterial pressure. These changes may be attributable to a direct depressant effect of the local anesthetic agent on various components of the cardiovascular system.
Pharmacokinetics and metabolism
Lidocaine may be absorbed following topical administration to mucous membranes, its rate and extent of absorption depending upon the specific site of application, duration of exposure, concentration, and total dosage. In general, the rate of absorption of local anesthetic agents following topical application occurs most rapidly after intratracheal administration. Lidocaine is also well-absorbed from the gastrointestinal tract, but little intact drug appears in the circulation because of biotransformation in the liver.
Lidocaine is metabolized rapidly by the liver, and metabolites and unchanged drug are excreted by the kidneys. Biotransformation includes oxidative N-dealkylation, ring hydroxylation, cleavage of the amide linkage, and conjugation. N-dealkylation, a major pathway of biotransformation, yields the metabolites monoethylglycinexylidide and glycinexylidide. The pharmacological/toxicological actions of these metabolites are similar to, but less potent than, those of lidocaine. Approximately 90% of lidocaine administered is excreted in the form of various metabolites, and less than 10% is excreted unchanged. The primary metabolite in urine is a conjugate of 4-hydroxy-2,6-dimethylaniline.
The plasma binding of lidocaine is dependent on drug concentration, and the fraction bound decreases with increasing concentration. At concentrations of 1 to 4 mcg of free base per mL, 60 to 80 percent of lidocaine is protein bound. Binding is also dependent on the plasma concentration of the alpha-l-acid glycoprotein.
Lidocaine crosses the blood-brain and placental barriers, presumably by passive diffusion.
Studies of lidocaine metabolism following intravenous bolus injections have shown that the elimination half-life of this agent is typically 1.5 to 2 hours. Because of the rapid rate at which lidocaine is metabolized, any condition that affects liver function may alter lidocaine kinetics. The half-life may be prolonged two-fold or more in patients with liver dysfunction. Renal dysfunction does not affect lidocaine kinetics but may increase the accumulation of metabolites.
Factors such as acidosis and the use of CNS stimulants and depressants affect the CNS levels of lidocaine required to produce overt systemic effects. Objective adverse manifestations become increasingly apparent with increasing venous plasma levels above 6 mcg free base per mL. In the rhesus monkey arterial blood levels of 18 to 21 mcg/mL have been shown to be threshold for convulsive activity.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
EXCESSIVE DOSAGE, OR SHORT INTERVALS BETWEEN DOSES, CAN RESULT IN HIGH PLASMA LEVELS AND SERIOUS ADVERSE EFFECTS, PATIENTS SHOULD BE INSTRUCTED TO STRICTLY ADHERE TO THE RECOMMENDED DOSAGE AND ADMINISTRATION GUIDELINES AS SET FORTH IN THIS PACKAGE INSERT.
THE MANAGEMENT OF SERIOUS ADVERSE REACTIONS MAY REQUIRE THE USE OF RESUSCITATIVE EQUIPMENT, OXYGEN, AND OTHER RESUSCITATIVE DRUGS.
Lidocaine Ointment 5% should be used with extreme caution in the presence of sepsis or severely traumatized mucosa in the area of application, since under such conditions there is the potential for rapid systemic absorption.
Methemoglobinemia
Cases of methemoglobinemia have been reported in association with local anesthetic use. Although all patients are at risk for methemoglobinemia, patients with glucose-6-phosphate dehydrogenase deficiency, congenital or idiopathic methemoglobinemia, cardiac or pulmonary compromise, infants under 6 months of age, and concurrent exposure to oxidizing agents or their metabolites are more susceptible to developing clinical manifestations of the condition. If local anesthetics must be used in these patients, close monitoring for symptoms and signs of methemoglobinemia is recommended.
Signs of methemoglobinemia may occur immediately or may be delayed some hours after exposure, and are characterized by a cyanotic skin discoloration and/or abnormal coloration of the blood. Methemoglobin levels may continue to rise; therefore, immediate treatment is required to avert more serious central nervous system and cardiovascular adverse effects, including seizures, coma, arrhythmias, and death. Discontinue Lidocaine Ointment 5% and any other oxidizing agents. Depending on the severity of the signs and symptoms, patients may respond to supportive care, i.e., oxygen therapy, hydration. A more severe clinical presentation may require treatment with methylene blue, exchange transfusion, or hyperbaric oxygen.
-
PRECAUTIONS
General
The safety and effectiveness of lidocaine depend on proper dosage, correct technique, adequate precautions, and readiness for emergencies (see WARNINGS and ADVERSE REACTIONS). The lowest dosage that results in effective anesthesia should be used to avoid high plasma levels and serious adverse effects. Repeated doses of lidocaine may cause significant increases in blood levels with each repeated dose because of slow accumulation of the drug and/or its metabolites. Tolerance to elevated blood levels varies with the status of the patient. Debilitated, elderly patients, acutely ill patients, and children should be given reduced doses commensurate with their age and physical condition. Lidocaine should also be used with caution in patients with severe shock or heart block.
Lidocaine Ointment 5% should be used with caution in patients with known drug sensitivities. Patients allergic to paraaminobenzoic acid derivatives (procaine, tetracaine, benzocaine, etc.) have not shown cross sensitivity to lidocaine. Many drugs used during the conduct of anesthesia are considered potential triggering agents for familial malignant hyperthermia. Since it is not known whether amide-type local anesthetics may trigger this reaction and since the need for supplemental general anesthesia cannot be predicted in advance, it is suggested that a standard protocol for the management of malignant hyperthermia should be available. Early unexplained signs of tachycardia, tachypnea, labile blood pressure and metabolic acidosis may precede temperature elevation. Successful outcome is dependent on early diagnosis, prompt discontinuance of the suspect triggering agent(s) and institution of treatment, including oxygen therapy, indicated supportive measures and dantrolene (consult dantrolene sodium intravenous package insert before using).
Drug Interactions
Patients who are administered local anesthetics are at increased risk of developing methemoglobinemia when concurrently exposed to the following drugs, which could include other local anesthetics:
Examples of Drugs Associated with Methemoglobinemia:
Class
Examples
Nitrates/Nitrites
nitroglycerin, nitroprusside, nitric oxide, nitrous oxide
Local anesthetics
benzocaine, lidocaine, bupivacaine, mepivacaine, tetracaine, prilocaine, procaine, articaine
Antineoplastic agents
cyclophosphamide, flutamide, rasburicase, ifosfamide, hydroxyurea
Antibiotics
dapsone, sulfonamides, nitrofurantoin,
para-aminosalicylic acidAntimalarials
chloroquine, primaquine
Anticonvulsants
phenytoin, sodium valproate, phenobarbital
Other drugs
acetaminophen, metoclopramide, sulfa drugs (i.e., sulfasalazine), quinine
Information for Patients
When topical anesthetics are used in the mouth, the patient should be aware that the production of topical anesthesia may impair swallowing and thus enhance the danger of aspiration. For this reason, food should not be ingested for 60 minutes following the use of local anesthetic preparations in the mouth or throat area. This is particularly important in children because of their frequency of eating.
Numbness of the tongue or buccal mucosa may enhance the danger of unintentional biting trauma. Food and chewing gum should not be taken while the mouth or throat area is anesthetized.
Inform patients that use of local anesthetics may cause methemoglobinemia, a serious condition that must be treated promptly. Advise patients or caregivers to seek immediate medical attention if they or someone in their care experience the following signs or symptoms: pale, gray, or blue colored skin (cyanosis); headache; rapid heart rate; shortness of breath; lightheadedness; or fatigue.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies of lidocaine in animals to evaluate the carcinogenic and mutagenic potential or the effect on fertility have not been conducted.
Use in Pregnancy
Teratogenic Effects. Pregnancy Category B. Reproduction studies have been performed in rats at doses up to 6.6 times the human dose and have revealed no evidence of harm to the fetus caused by lidocaine. There are, however, no adequate and well-controlled studies in pregnant women. Animal reproduction studies are not always predictive of human response. General consideration should be given to this fact before administering lidocaine to women of childbearing potential, especially during early pregnancy when maximum organogenesis takes place.
Labor and Delivery
Lidocaine is not contraindicated in labor and delivery. Should Lidocaine Ointment 5% be used concomitantly with other products containing lidocaine, the total dose contributed by all formulations must be kept in mind.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when lidocaine is administered to a nursing woman.
Pediatric Use
Dosage in children should be reduced, commensurate with age, body weight and physical condition. Caution must be taken to avoid overdosage when applying Lidocaine Ointment 5% to large areas of injured or abraded skin, since the systemic absorption of lidocaine may be increased under such conditions (see DOSAGE AND ADMINISTRATION).
-
ADVERSE REACTIONS
Adverse experiences following the administration of lidocaine are similar in nature to those observed with other amide local anesthetic agents. These adverse experiences are, in general, dose-related and may result from high plasma levels caused by excessive dosage or rapid absorption, or may result from a hypersensitivity, idiosyncrasy or diminished tolerance on the part of the patient. Serious adverse experiences are generally systemic in nature. The following types are those most commonly reported:
Central nervous system
CNS manifestations are excitatory and/or depressant and may be characterized by lightheadedness, nervousness, apprehension, euphoria, confusion, dizziness, drowsiness, tinnitus, blurred or double vision, vomiting, sensations of heat, cold or numbness, twitching, tremors, convulsions, unconsciousness, respiratory depression and arrest. The excitatory manifestations may be very brief or may not occur at all, in which case the first manifestation of toxicity may be drowsiness merging into unconsciousness and respiratory arrest. Drowsiness following the administration of lidocaine is usually an early sign of a high blood level of the drug and may occur as a consequence of rapid absorption.
Cardiovascular system
Cardiovascular manifestations are usually depressant and are characterized by bradycardia, hypotension, and cardiovascular collapse, which may lead to cardiac arrest.
Allergic
Allergic reactions are characterized by cutaneous lesions, urticaria, edema or anaphylactoid reactions. Allergic reactions may occur as a result of sensitivity either to the local anesthetic agent or to other components in the formulation. Allergic reactions as a result of sensitivity to lidocaine are extremely rare and, if they occur, should be managed by conventional means. The detection of sensitivity by skin testing is of doubtful value.
To report SUSPECTED ADVERSE REACTIONS, contact Amneal Pharmaceuticals at 1-877-835-5472 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
-
OVERDOSAGE
Acute emergencies from local anesthetics are generally related to high plasma levels encountered during therapeutic use of local anesthetics (see ADVERSE REACTIONS, WARNINGS, and PRECAUTIONS).
Management of local anesthetic emergencies
The first consideration is prevention, best accomplished by careful and constant monitoring of cardiovascular and respiratory vital signs and the patient's state of consciousness after each local anesthetic administration. At the first sign of change, oxygen should be administered.
The first step in the management of convulsions consists of immediate attention to the maintenance of a patent airway and assisted or controlled ventilation with oxygen and a delivery system capable of permitting immediate positive airway pressure by mask. Immediately after the institution of these ventilatory measures, the adequacy of the circulation should be evaluated, keeping in mind that drugs used to treat convulsions sometimes depress the circulation when administered intravenously. Should convulsions persist despite adequate respiratory support, and if the status of the circulation permits, small increments of an ultra-short acting barbiturate (such as thiopental or thiamylal) or a benzodiazepine (such as diazepam) may be administered intravenously. The clinician should be familiar, prior to use of local anesthetics, with these anticonvulsant drugs. Supportive treatment of circulatory depression may require administration of intravenous fluids and, when appropriate, a vasopressor as directed by the clinical situation (e.g., ephedrine).
If not treated immediately, both convulsions and cardiovascular depression can result in hypoxia, acidosis, bradycardia, arrhythmias and cardiac arrest. If cardiac arrest should occur, standard cardiopulmonary resuscitative measures should be instituted.
Dialysis is of negligible value in the treatment of acute overdosage with lidocaine.
The oral LD50 of lidocaine HCI in non-fasted female rats is 459 (346 to 773) mg/kg (as the salt) and 214 (159 to 324) mg/kg (as the salt) in fasted female rats.
-
DOSAGE AND ADMINISTRATION
When Lidocaine Ointment 5% is used concomitantly with other products containing lidocaine, the total dose contributed by all formulations must be kept in mind.
Adult
A single application should not exceed 5 g of Lidocaine Ointment 5%, containing 250 mg of lidocaine base (equivalent chemically to approximately 300 mg of lidocaine hydrochloride). This is roughly equivalent to squeezing a six (6) inch length of ointment from the tube. In a 70 kg adult this dose equals 3.6 mg/kg (1.6 mg/lb) lidocaine base. No more than one-half tube, approximately 17 g to 20 g of ointment or 850 mg to 1000 mg lidocaine base, should be administered in any one day.
Although the incidence of adverse effects with Lidocaine Ointment 5% is quite low, caution should be exercised, particularly when employing large amounts, since the incidence of adverse effects is directly proportional to the total dose of local anesthetic agent administered.
Dosage for children
It is difficult to recommend a maximum dose of any drug for children since this varies as a function of age and weight. For children less than ten years who have a normal lean body mass and a normal lean body development, the maximum dose may be determined by the application of one of the standard pediatric drug formulas (e.g., Clark's rule). For example a child of five years weighing 50 lbs., the dose of lidocaine should not exceed 75 mg to 100 mg when calculated according to Clark's rule. In any case, the maximum amount of lidocaine administered should not exceed 4.5 mg/kg (2 mg/lb) of body weight.
Administration
For medical use, apply topically for adequate control of symptoms. The use of a sterile gauze pad is suggested for application to broken skin tissue. Apply to the tube prior to intubation.
In dentistry, apply to previously dried oral mucosa. Subsequent removal of excess saliva with cotton rolls or saliva ejector minimizes dilution of the ointment, permits maximum penetration, and minimizes the possibility of swallowing the topical ointment.
For use in connection with the insertion of new dentures, apply to all denture surfaces contacting mucosa.
IMPORTANT: Patients should consult a dentist at intervals not exceeding 48 hours throughout the fitting period.
-
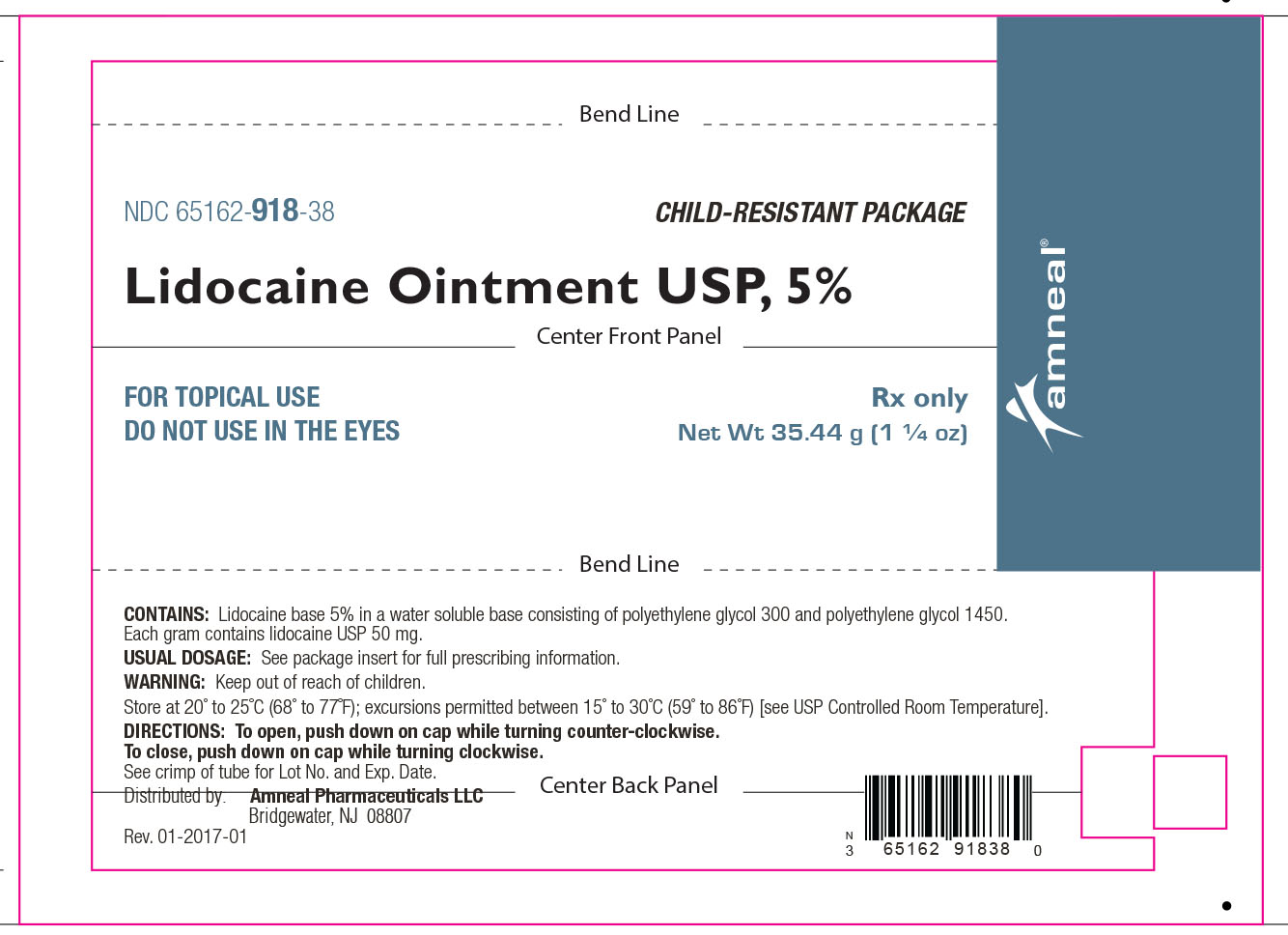
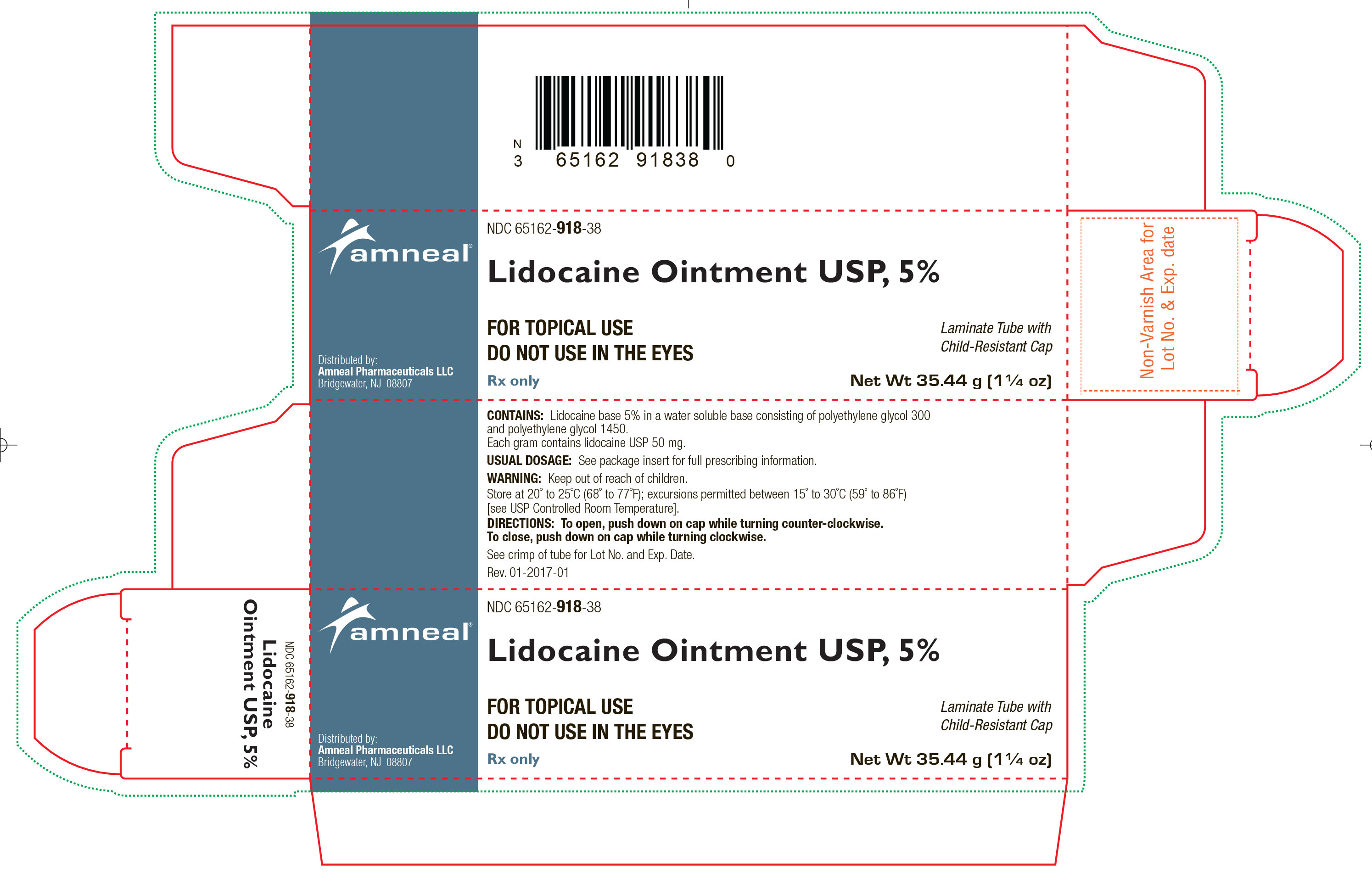
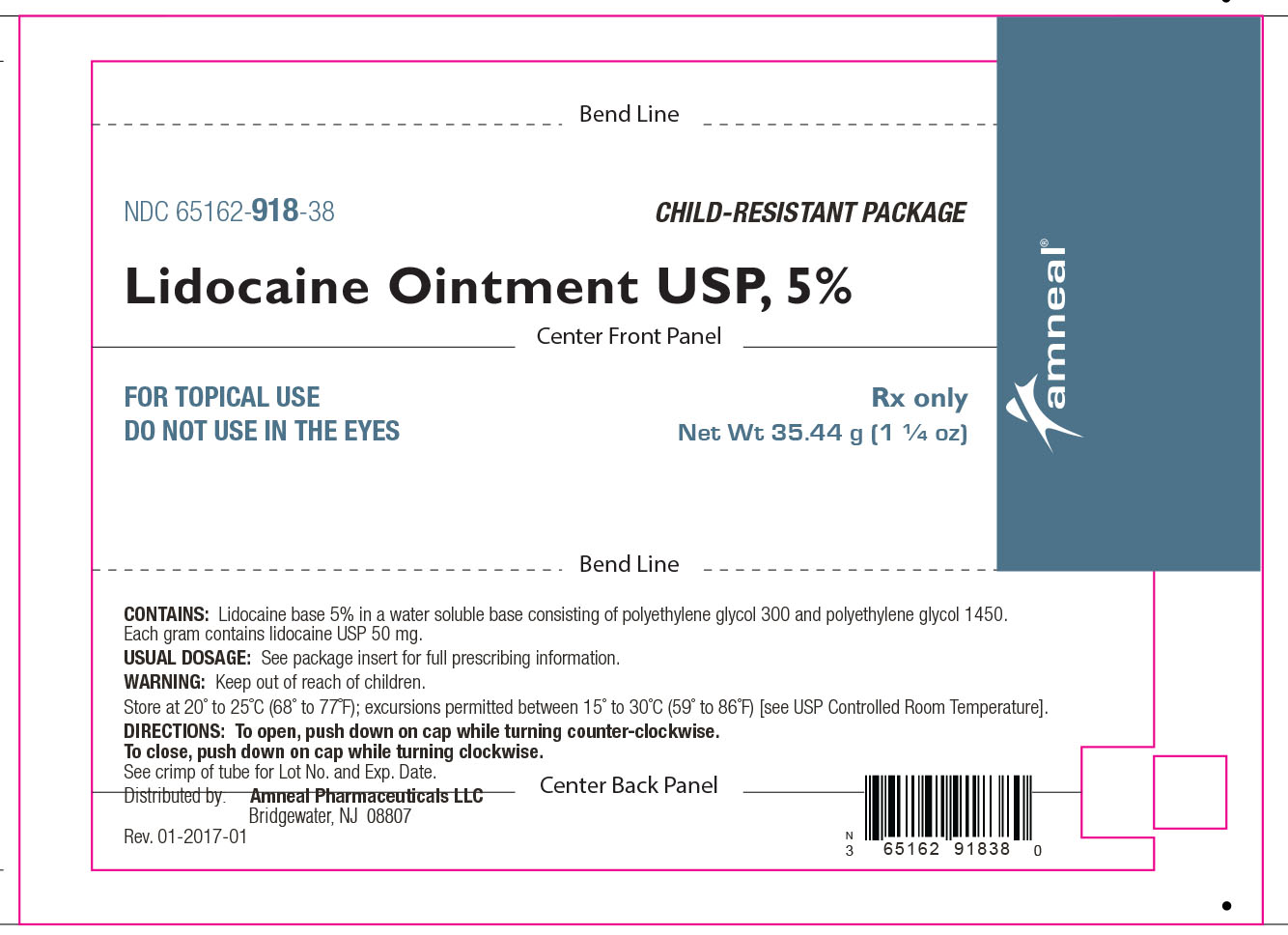
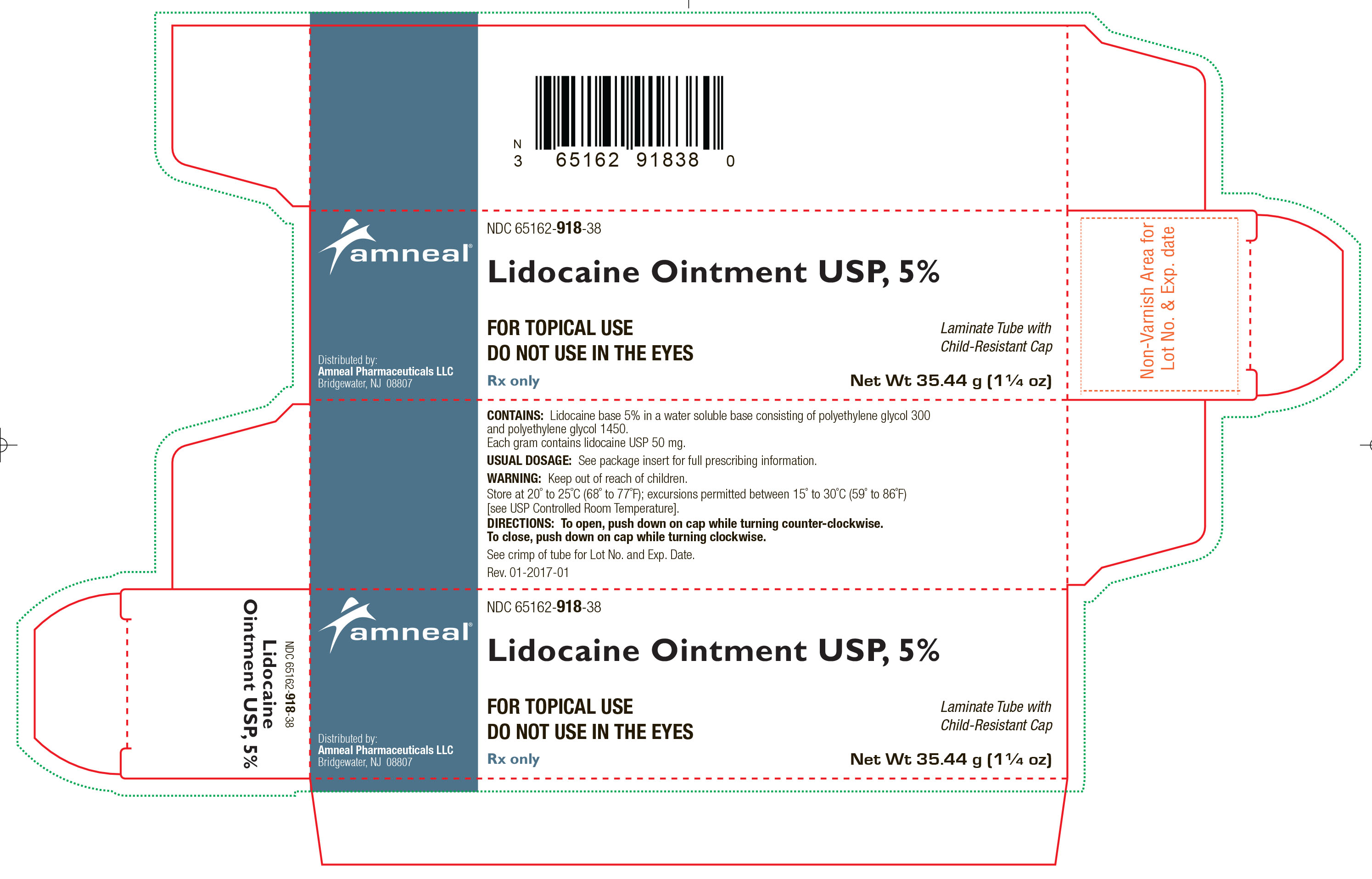
HOW SUPPLIED
Lidocaine Ointment USP, 5% is supplied as a white to off-white color ointment.
It is available as follows:
30 g laminate tubes with a child-resistant cap, NDC 65162-918-30.
35.44 g (1 ¼ oz) laminate tubes with a child-resistant cap, NDC 65162-918-38.
50 g (1 ¾ oz) double wall jar, with a child-resistant cap, NDC 65162-918-53.
Pharmacist: If dispensed to a consumer, provide child resistant package for dispensing.
Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Distributed by:
Amneal Pharmaceuticals LLC
Bridgewater, NJ 08807
Rev. 10-2018-08
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LIDOCAINE
lidocaine ointmentProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:65162-918 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 50 mg in 1 g Inactive Ingredients Ingredient Name Strength POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) Product Characteristics Color white (white to off-white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65162-918-30 1 in 1 CARTON 08/10/2015 1 30 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:65162-918-38 1 in 1 CARTON 08/10/2015 2 35.44 g in 1 TUBE; Type 0: Not a Combination Product 3 NDC:65162-918-53 50 g in 1 JAR; Type 0: Not a Combination Product 08/10/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA206297 08/10/2015 Labeler - Amneal Pharmaceuticals LLC (123797875) Establishment Name Address ID/FEI Business Operations Amneal Pharmaceuticals, LLC 053542455 analysis(65162-918) , label(65162-918) , manufacture(65162-918) , pack(65162-918)