Label: AZELASTINE HYDROCHLORIDE spray, metered
-
Contains inactivated NDC Code(s)
NDC Code(s): 65162-706-84 - Packager: Amneal Pharmaceuticals LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 27, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use AZELASTINE HYDROCHLORIDE NASAL SPRAY safely and effectively. See full prescribing information for AZELASTINE HYDROCHLORIDE NASAL ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE
Azelastine HCl nasal spray, 0.15% is indicated for the relief of the symptoms of seasonal allergic rhinitis in patients 6 years of age and older and perennial allergic rhinitis in patients 6 years ...
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage - for Seasonal Allergic Rhinitis - Children 6 to 11 years of age: Azelastine HCl nasal spray, 0.15%, 1 spray per nostril twice daily. Adults and adolescents 12 years of ...
-
3 DOSAGE FORMS AND STRENGTHS
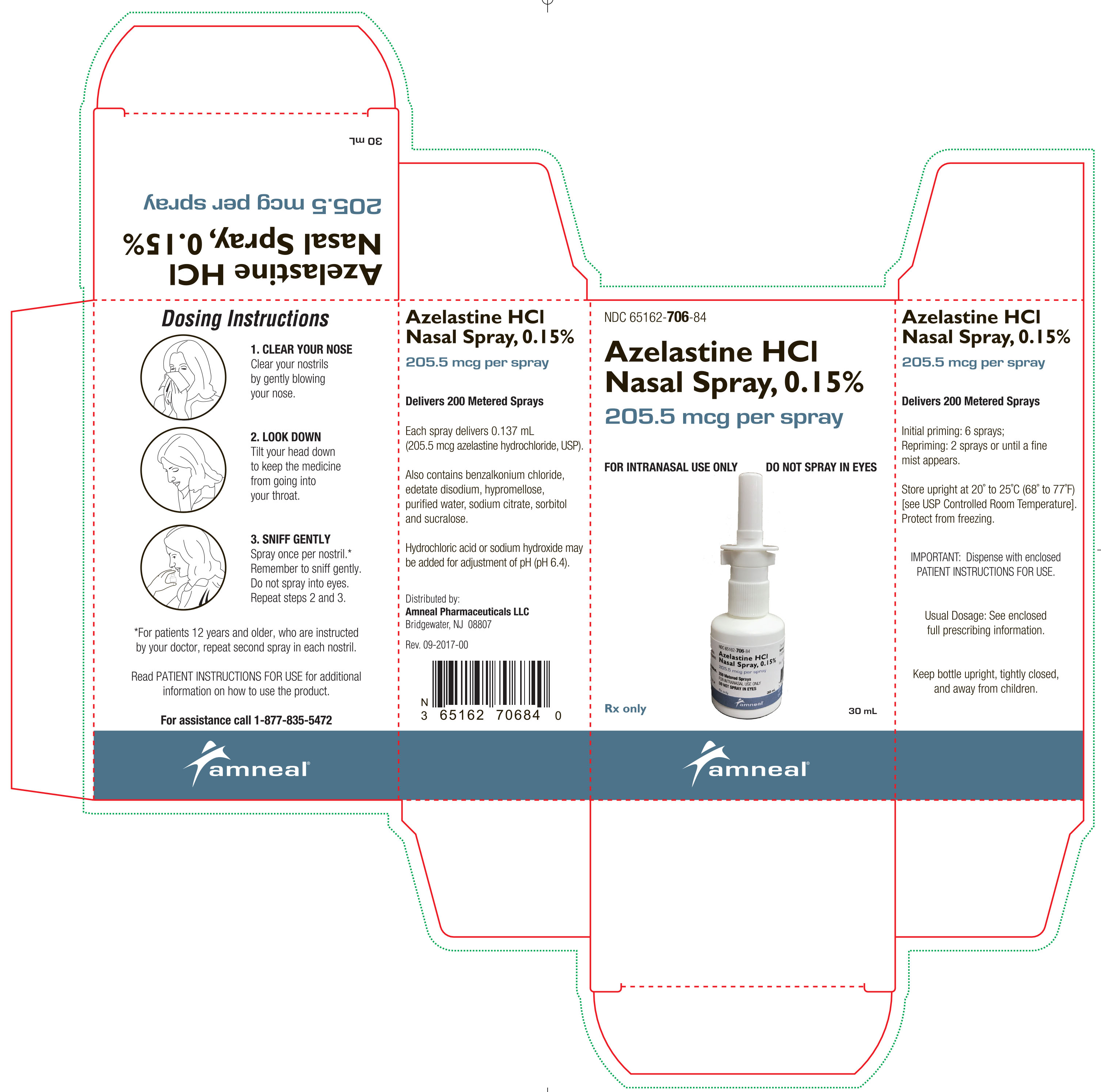
Azelastine HCl nasal spray, 0.15% is a nasal spray solution available in one dosage strength: Each spray of azelastine HCl nasal spray, 0.15% delivers a volume of 0.137 mL solution containing ...
-
4 CONTRAINDICATIONS
None.
-
5 WARNINGS AND PRECAUTIONS
5.1 Somnolence - In clinical trials, the occurrence of somnolence has been reported in some patients taking azelastine HCl nasal spray, 0.15% [see Adverse Reactions (6.1)]. Patients should be ...
-
6 ADVERSE REACTIONS
The following clinically significant adverse reaction is described elsewhere in the labeling: Somnolence [see Warnings and Precautions (5.1)] 6.1 Clinical Trials Experience - Because clinical ...
-
7 DRUG INTERACTIONS
7.1 Central Nervous System Depressants - Concurrent use of azelastine HCl nasal spray, 0.15% with alcohol or other central nervous system depressants should be avoided because reductions in ...
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Risk Summary - Limited data from post-marketing experience over decades of use with azelastine HCl in pregnant women have not identified any drug associated risks of miscarriage ...
-
11 DESCRIPTION
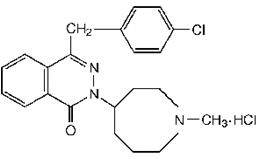
Azelastine HCl nasal spray, 0.15% is an antihistamine (H1 receptor antagonist) formulated as a metered-spray solution for intranasal administration. Azelastine HCl, USP occurs as a white, almost ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - Azelastine HCl, a phthalazinone derivative, exhibits histamine H1-receptor antagonist activity in isolated tissues, animal models, and humans. Azelastine HCl nasal ...
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Two-year carcinogenicity studies in Crl:CD(SD)BR rats and NMRI mice, were conducted to assess the carcinogenic potential of ...
-
14 CLINICAL STUDIES
14.1 Seasonal Allergic Rhinitis - Azelastine HCl Nasal Spray, 0.15% The efficacy and safety of azelastine HCl nasal spray, 0.15% in seasonal allergic rhinitis was evaluated in five ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING
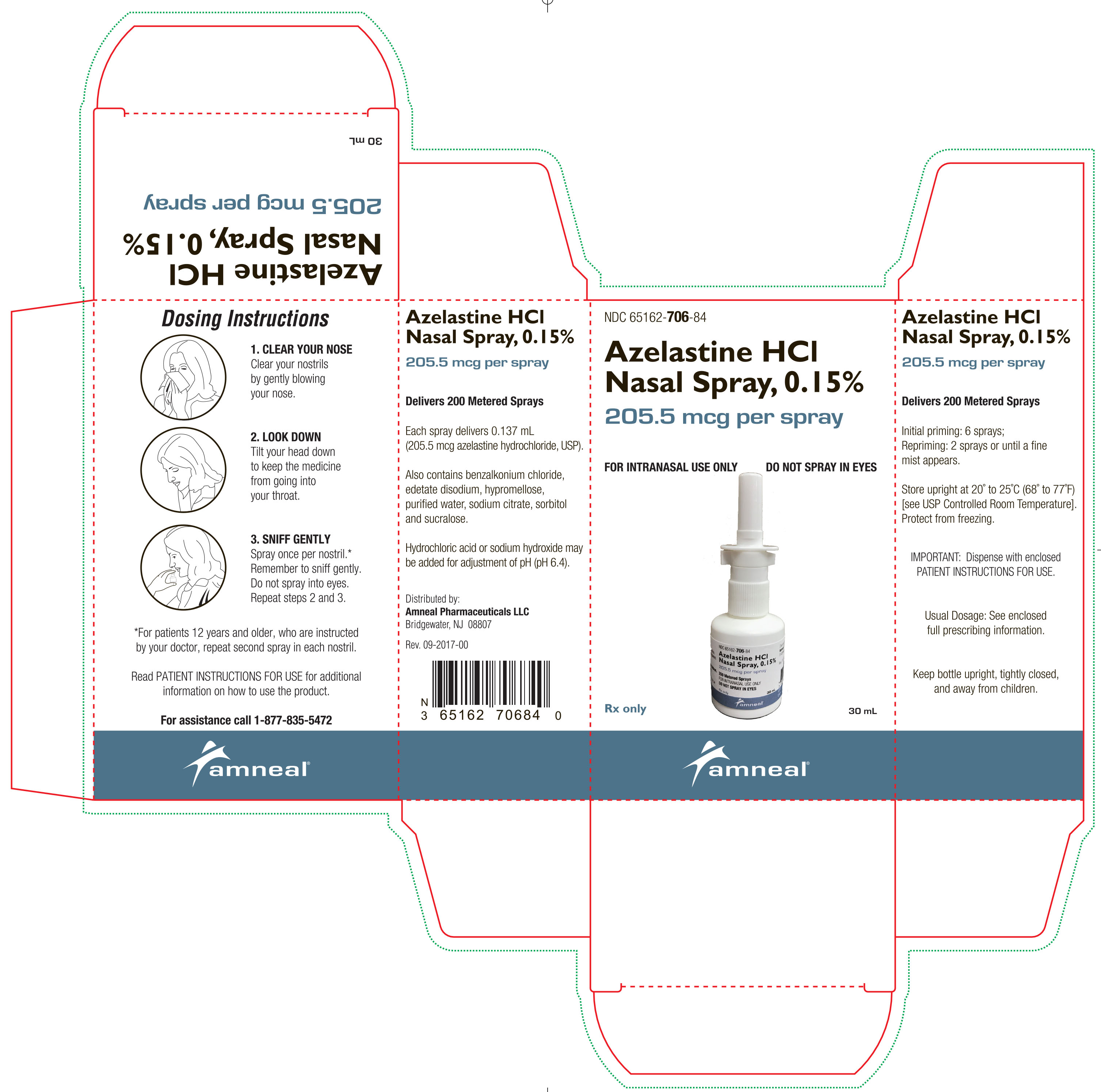
Azelastine HCl nasal spray, 0.15% is supplied as a 30 mL package (NDC 65162-706-84) delivering 200 metered sprays in a high-density polyethylene (HDPE) bottle fitted with a metered-dose spray pump ...
-
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Patient Information and Instructions for Use). Somnolence - Somnolence has been reported in some patients taking azelastine HCl nasal spray, 0.15%. Caution ...
-
PATIENT INFORMATION
Azelastine (ay” ze las’ teen) HCl Nasal Spray, 0.15% Important: For use in your nose only. What is Azelastine HCl Nasal Spray, 0.15%? Azelastine HCl nasal spray, 0.15% is a ...
-
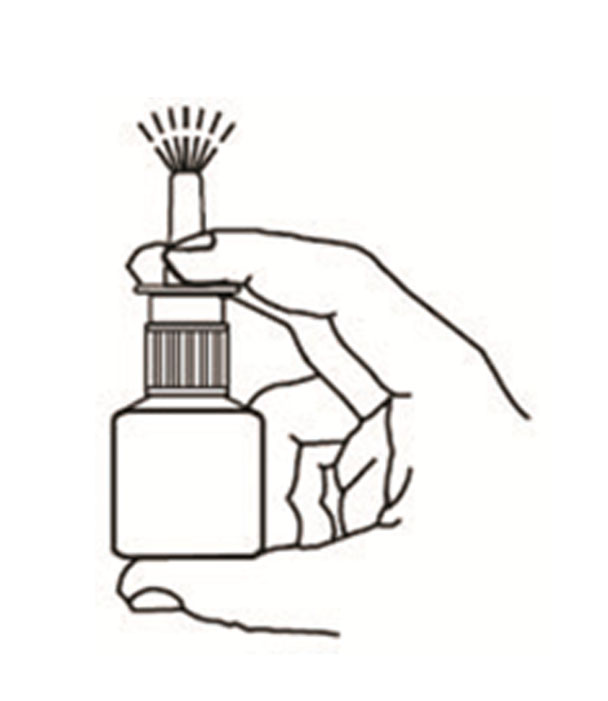
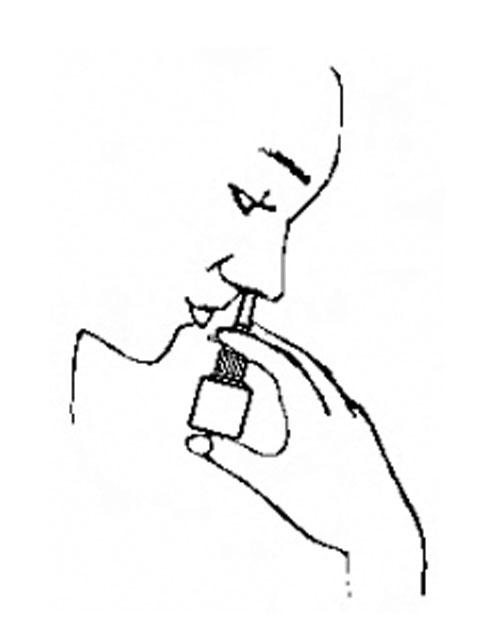
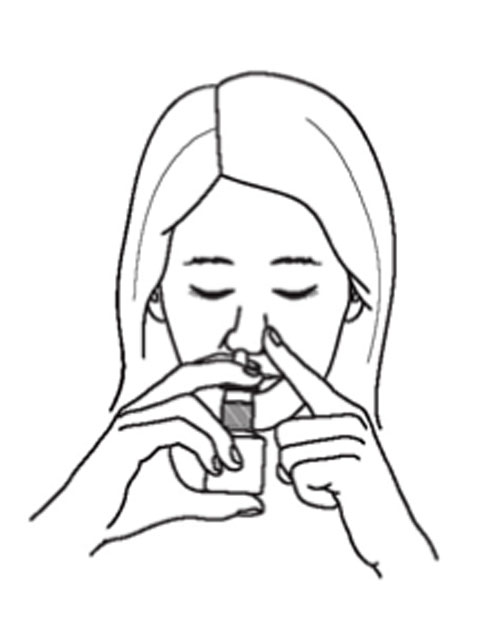
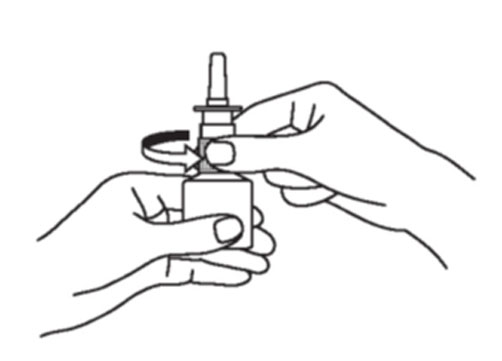
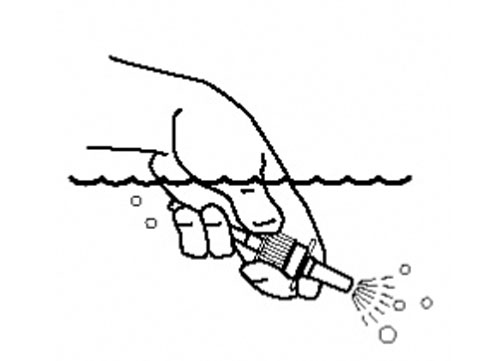
Instructions for Use
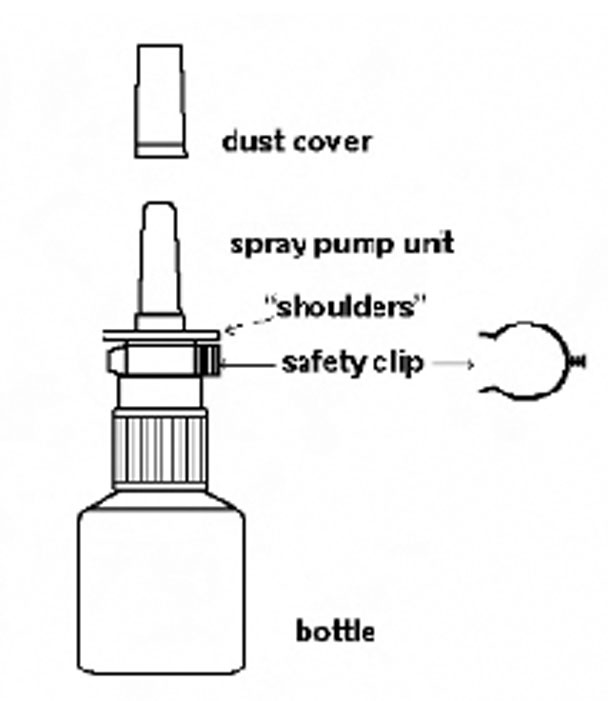
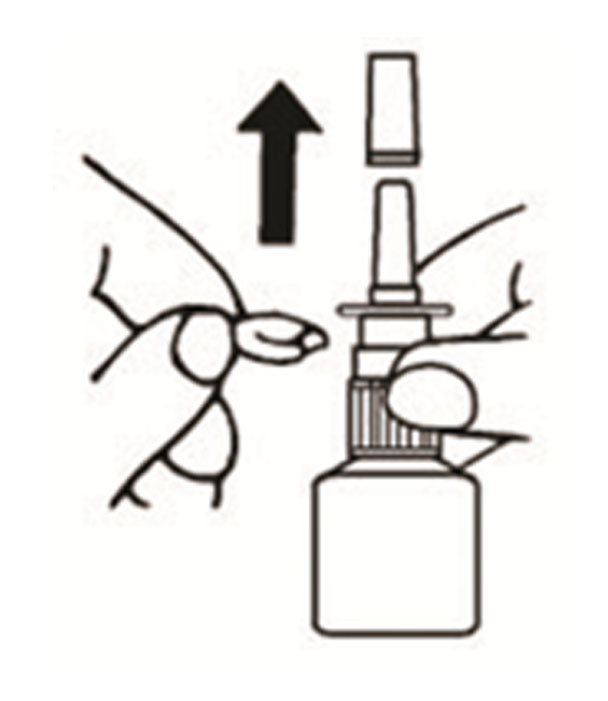
Azelastine (ay” ze las’ teen) Hydrochloride Nasal Spray, 0.15% Important: For use in your nose only. For the correct dose of medicine: Keep your head tilted downward when ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
 ...
... -
INGREDIENTS AND APPEARANCEProduct Information