Label: FLUOCINOLONE ACETONIDE oil
- NDC Code(s): 65162-704-86
- Packager: Amneal Pharmaceuticals LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated August 25, 2015
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use FLUOCINOLONE ACETONIDE 0.01% TOPICAL OIL safely and effectively. See full prescribing information for FLUOCINOLONE ACETONIDE 0.01 ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Adult Patients with Atopic Dermatitis - Fluocinolone Acetonide 0.01% Topical Oil is indicated for the topical treatment of atopic dermatitis in adult patients. 1.2 Pediatric ...
-
2 DOSAGE AND ADMINISTRATIONFluocinolone acetonide 0.01% topical oil is not for oral, ophthalmic, or intravaginal use. The dosing of fluocinolone acetonide 0.01% topical oil is different for adult and pediatric ...
-
3 DOSAGE FORMS AND STRENGTHSFluocinolone Acetonide 0.01% Topical Oil (Body Oil) is supplied in 4 fluid ounce bottles with a net content of 118.28 mL.
-
4 CONTRAINDICATIONSNone
-
5 WARNINGS AND PRECAUTIONS5.1 Hypothalamic-Pituitary-Adrenal Axis Suppression - Systemic absorption of topical corticosteroids can produce reversible hypothalamic-pituitary-adrenal (HPA) axis suppression with ...
-
6 ADVERSE REACTIONSBecause clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Pregnancy Category C: Corticosteroids have been shown to be teratogenic in laboratory animals when administered systemically at relatively low dosage levels. Some corticosteroids ...
-
10 OVERDOSAGETopically applied corticosteroids can be absorbed in sufficient amounts to produce systemic effects, including under conditions of normal use [see Warnings and Precautions (5.1) and Use in ...
-
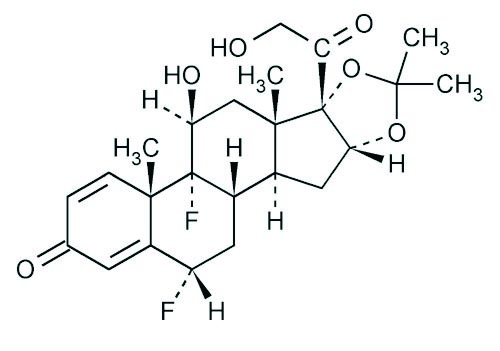
11 DESCRIPTIONFluocinolone Acetonide 0.01% Topical Oil (Body Oil) contains fluocinolone acetonide [(6α, 11β, 16α)-6,9-difluoro-11,21-dihydroxy-16,17[(1-methylethylidene)bis(oxy)]-pregna-1,4-diene-3,20-dione ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Like other topical corticosteroids, fluocinolone acetonide has anti-inflammatory, antipruritic, and vasoconstrictive properties. The mechanism of the anti-inflammatory ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis and Mutagenesis and Impairment of Fertility - Long-term animal studies have not been performed to evaluate the carcinogenic potential or the effect on fertility of ...
-
16 HOW SUPPLIED / STORAGE AND HANDLINGFluocinolone Acetonide 0.01% Topical Oil (Body Oil) is supplied in 4 fluid ounce bottles with a net content of 118.28 mL (NDC 65162-704-86). Storage: Store upright at 20° to 25°C (68° to 77°F) ...
-
17 PATIENT COUNSELING INFORMATION17.1 Instructions - Fluocinolone acetonide 0.01% topical oil should be used as directed by the physician. It is for external use only. Avoid contact with the eyes. In case of contact ...
-
PRINCIPAL DISPLAY PANEL

-
INGREDIENTS AND APPEARANCEProduct Information