Label: RANITIDINE tablet
- NDC Code(s): 65162-253-06, 65162-253-10, 65162-253-11, 65162-253-18, view more
- Packager: Amneal Pharmaceuticals LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 30, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
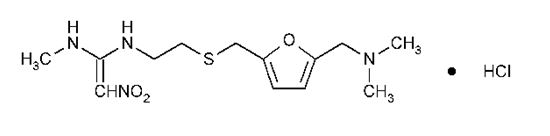
DESCRIPTIONThe active ingredient in Ranitidine Tablets USP, 150 mg and Ranitidine Tablets USP, 300 mg is ranitidine hydrochloride (HCl), USP, a histamine H2-receptor antagonist. Chemically it is ...
-
CLINICAL PHARMACOLOGYRanitidine Tablets are a competitive, reversible inhibitor of the action of histamine at the histamine H2-receptors, including receptors on the gastric cells. Ranitidine Tablets do not lower serum ...
-
CLINICAL TRIALSActive Duodenal Ulcer: In a multicenter, double-blind, controlled, US study of endoscopically diagnosed duodenal ulcers, earlier healing was seen in the patients treated with Ranitidine Tablets ...
-
INDICATIONS AND USAGERanitidine Tablets are indicated in: 1. Short-term treatment of active duodenal ulcer. Most patients heal within 4 weeks. Studies available to date have not assessed the safety of ranitidine in ...
-
CONTRAINDICATIONSRanitidine Tablets are contraindicated for patients known to have hypersensitivity to the drug or any of the ingredients (see PRECAUTIONS).
-
PRECAUTIONSGeneral - 1. Symptomatic response to therapy with Ranitidine Tablets does not preclude the presence of gastric malignancy. 2. Since ranitidine is excreted primarily by the kidney, dosage ...
-
ADVERSE REACTIONSThe following have been reported as events in clinical trials or in the routine management of patients treated with Ranitidine Tablets. The relationship to therapy with Ranitidine Tablets has been ...
-
OVERDOSAGEThere has been limited experience with overdosage. Reported acute ingestions of up to 18 g orally have been associated with transient adverse effects similar to those encountered in normal ...
-
DOSAGE AND ADMINISTRATIONActive Duodenal Ulcer: The current recommended adult oral dosage of Ranitidine Tablets for duodenal ulcer is 150 mg twice daily. An alternative dosage of 300 mg once daily after the evening meal ...
-
HOW SUPPLIEDRanitidine Tablets USP, 150 mg (ranitidine HCl equivalent to 150 mg of ranitidine) are supplied as orange, round, biconvex aqueous film-coated tablets debossed “IP 253” on one side and plain on ...
-
PRINCIPAL DISPLAY PANELNDC 65162-253-06 - Ranitidine Tablets USP, 150 mg - Rx only - 60 Tablets - Amneal Pharmaceuticals - Label (Matoda) NDC 65162-254-03 - Ranitidine Tablets USP, 300 mg - Rx only - 30 ...
-
INGREDIENTS AND APPEARANCEProduct Information