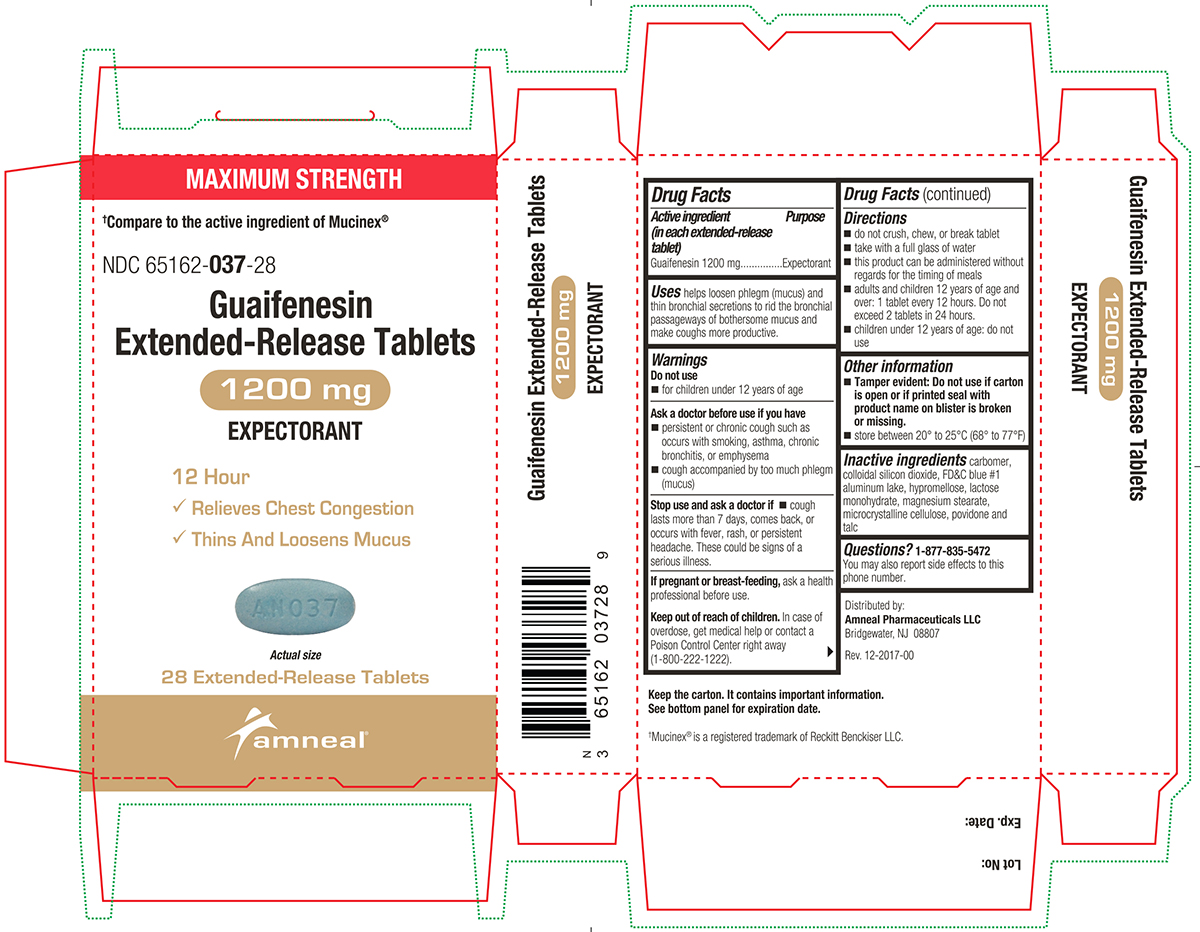
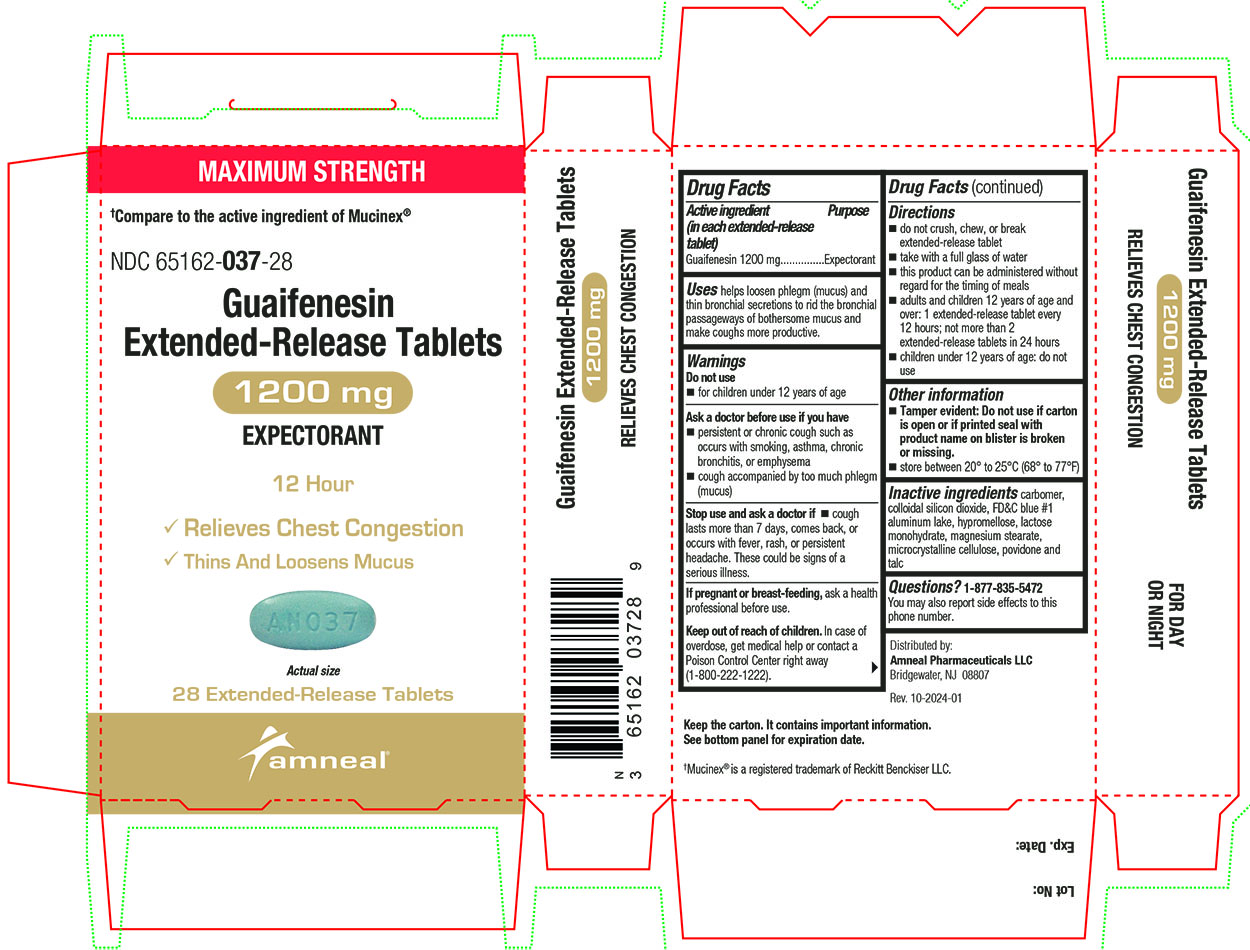
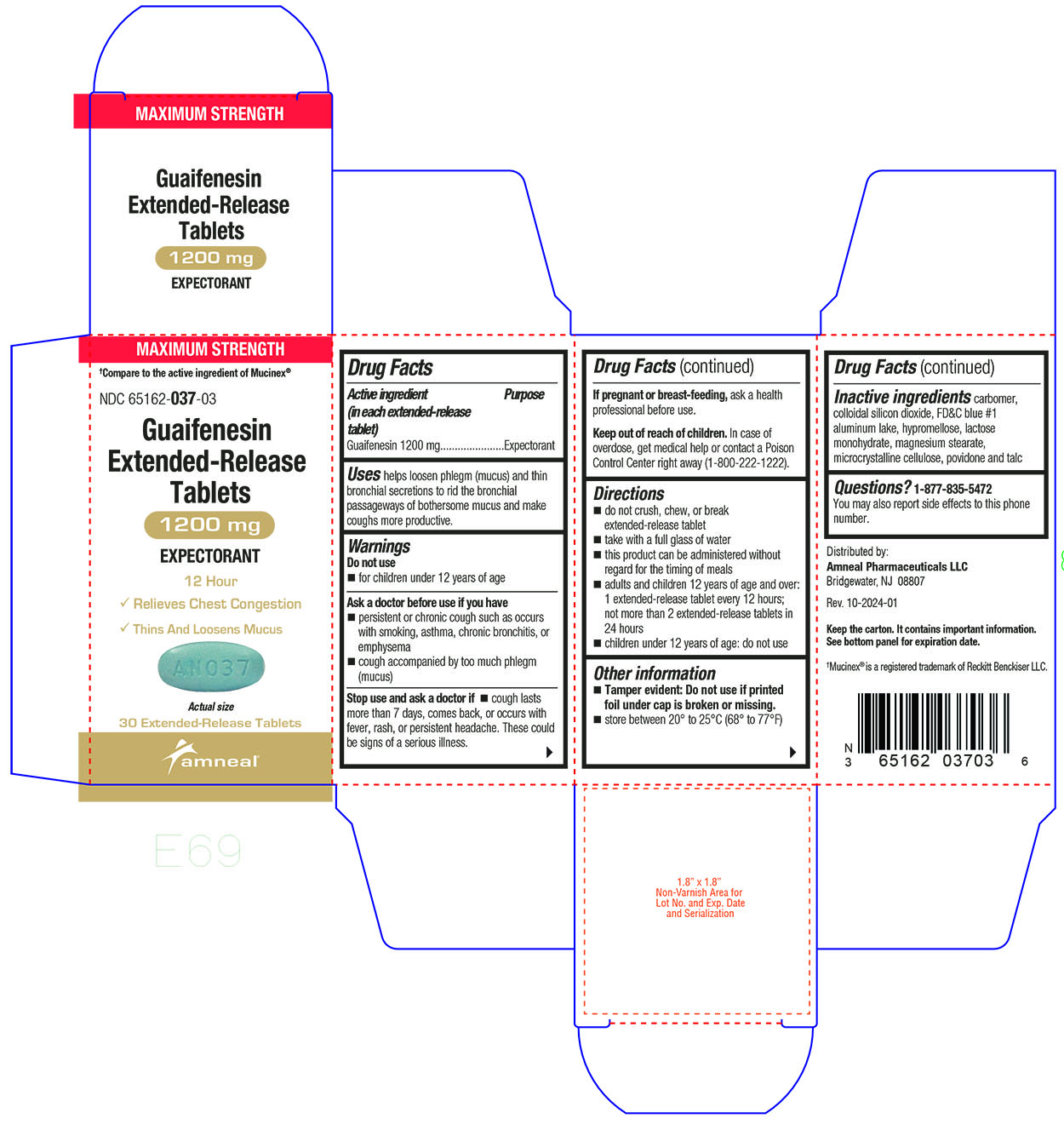
Label: GUAIFENESIN tablet, extended release
- NDC Code(s): 65162-036-02, 65162-036-03, 65162-036-06, 65162-037-03, view more
- Packager: Amneal Pharmaceuticals LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 7, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
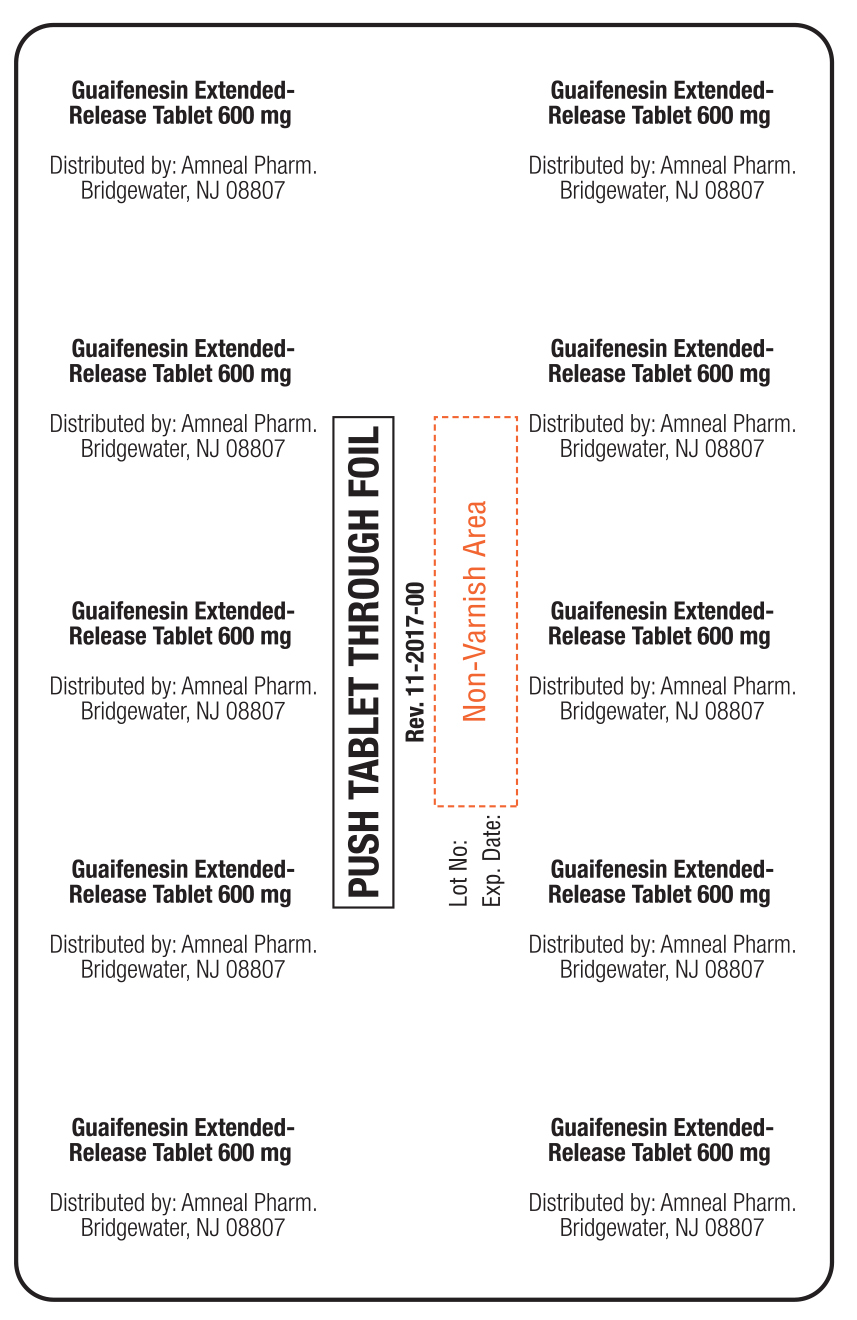
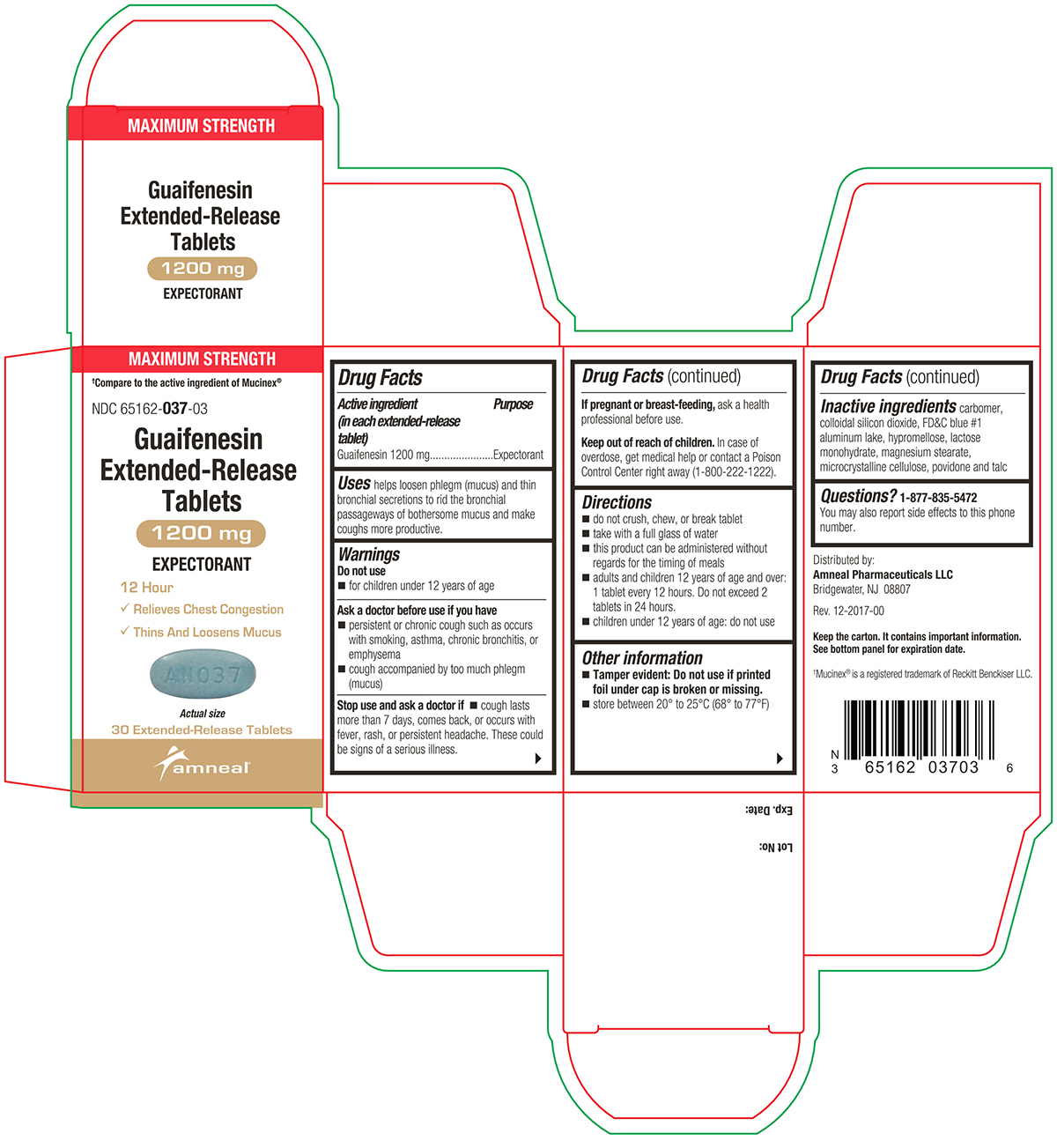
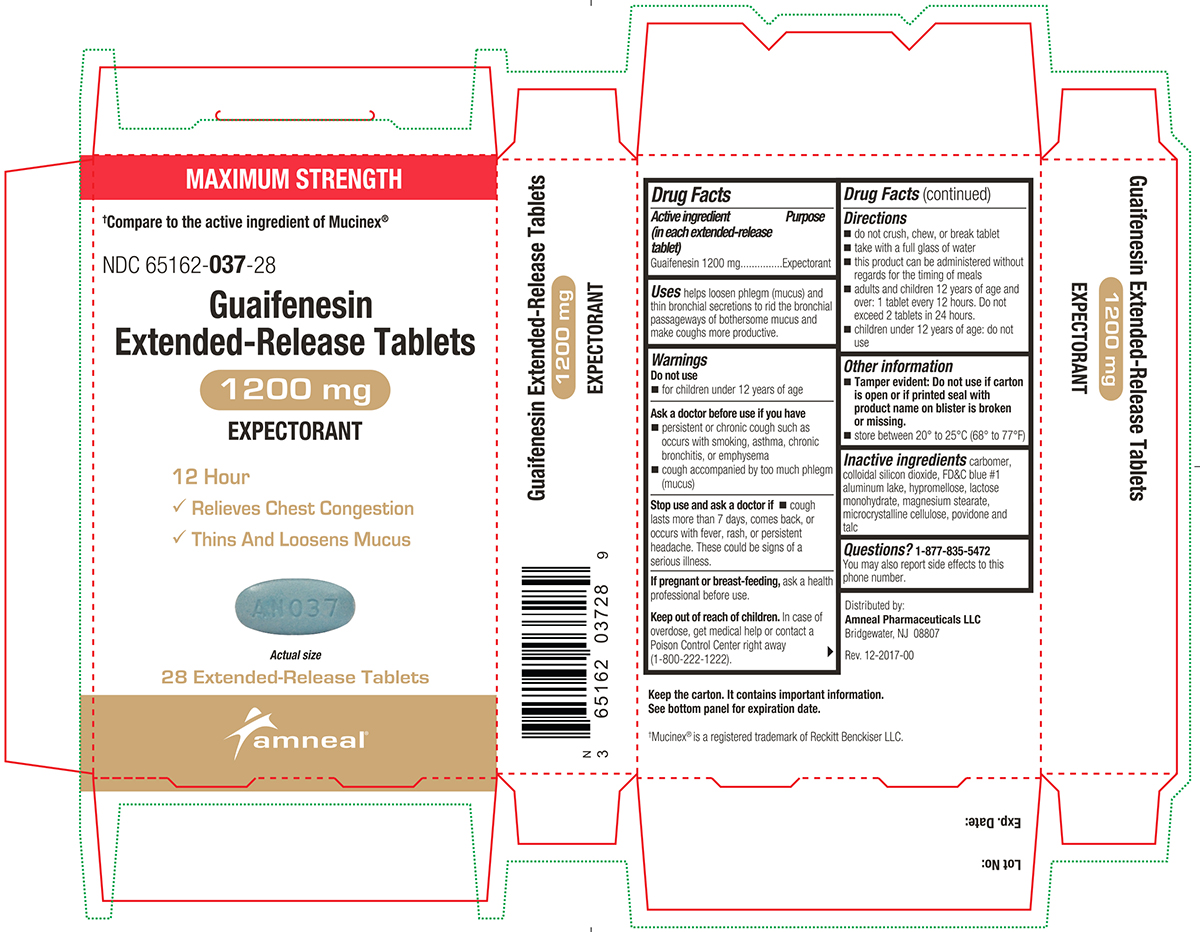
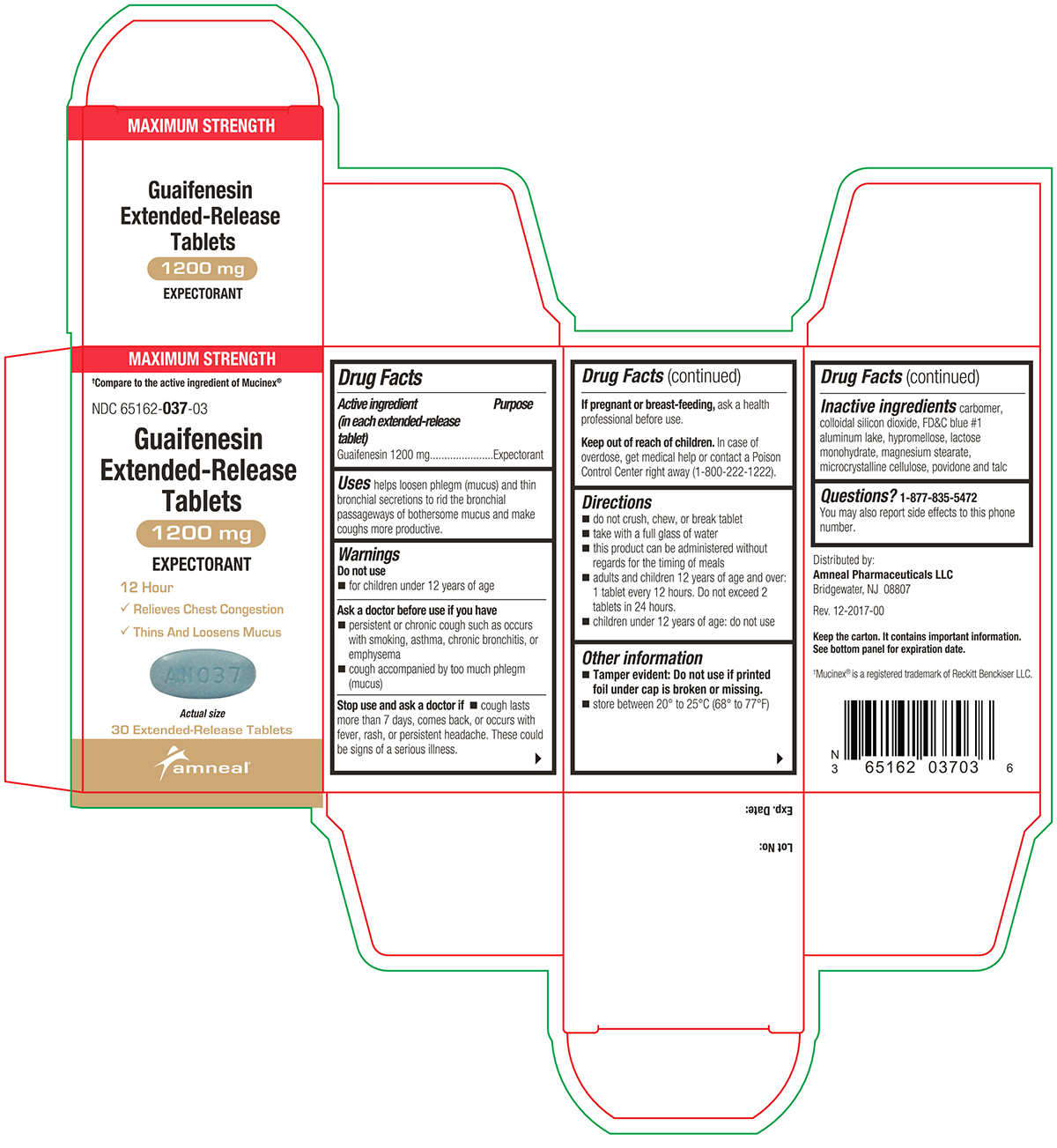
Active ingredient(s)Active ingredient (in each extended-release tablet) Guaifenesin 600 mg - Active ingredient (in each extended-release tablet) Guaifenesin 1200 mg
-
Purpose
Expectorant
-
Uses
helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive.
-
Warnings
Do not use - for children under 12 years of age - Ask a doctor before use if you have - persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema ...
-
Directions
600 mg - do not crush, chew, or break extended-release tablet - take with a full glass of water - this product can be administered without regard for the timing of meals - adults and children 12 years ...
-
Other information
Tamper evident: Do not use if printed foil under cap is broken or missing.
-
Storagestore between 20° to 25°C (68° to 77°F)
-
Inactive ingredients
carbomer, colloidal silicon dioxide, FD&C blue #1 aluminum lake, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, povidone and talc
-
Questions1-877-835-5472 You may also report side effects to this phone number. Distributed by: Amneal Pharmaceuticals LLC - Bridgewater, NJ 08807 - Rev. 10-2024-01
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
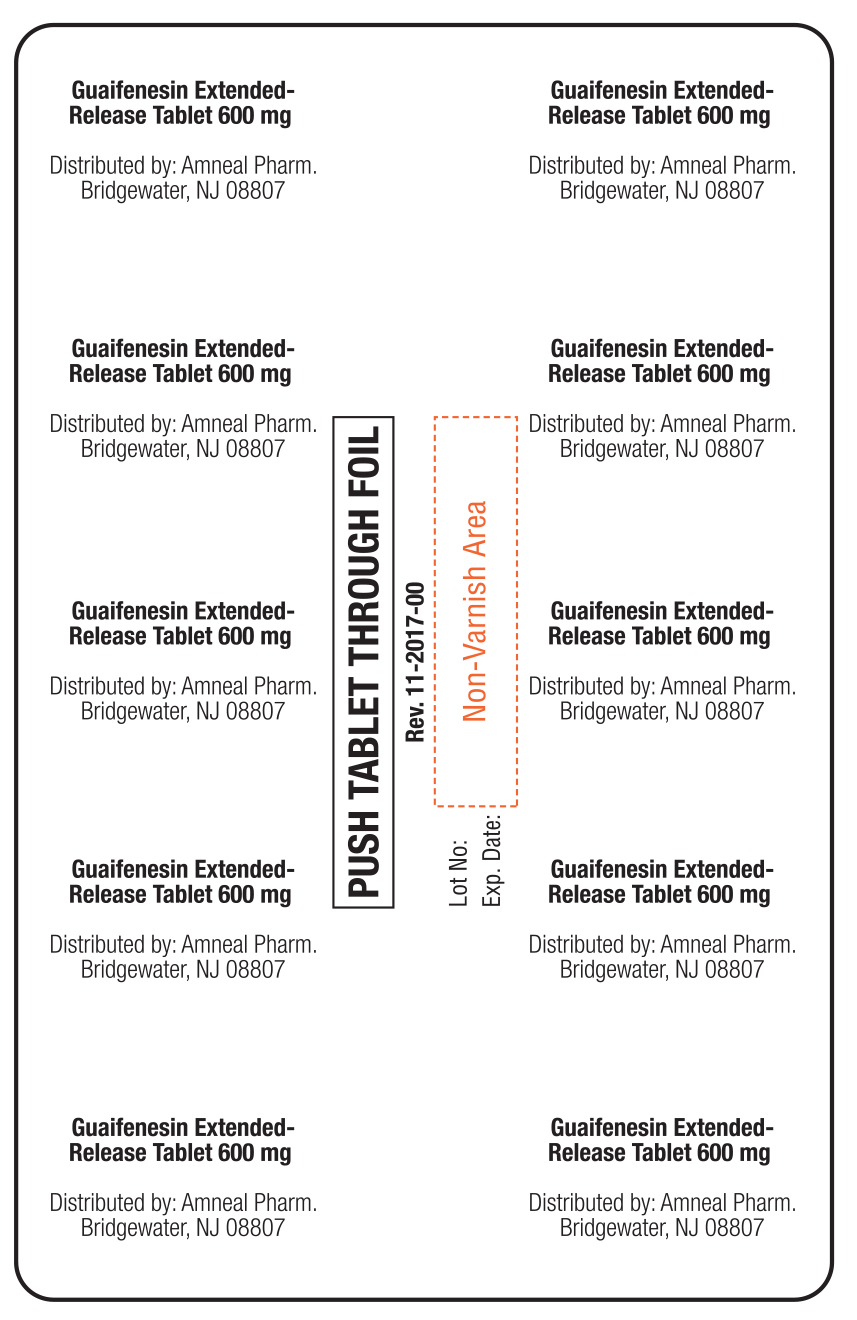 ...
... -
INGREDIENTS AND APPEARANCEProduct Information