Label: DAPSONE tablet
- NDC Code(s): 64980-566-01, 64980-566-03, 64980-567-01, 64980-567-03
- Packager: Rising Pharma Holdings, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated August 29, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
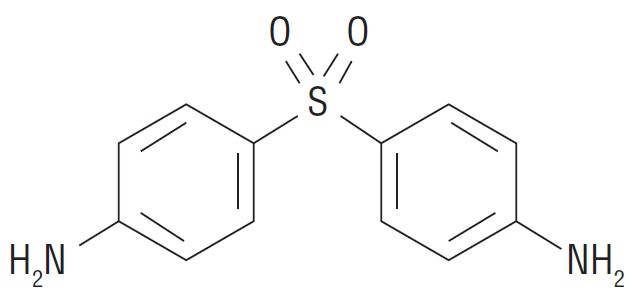
DESCRIPTIONDapsone-USP, 4,4’-sulfonyl dianiline, is a primary treatment for Dermatitis herpetiformis. It is an antibacterial drug for susceptible cases of leprosy. It is a white to creamy white crystalline ...
-
CLINICAL PHARMACOLOGYActions: The mechanism of action in Dermatitis herpetiformis has not been established. By the kinetic method in mice, Dapsone is bactericidal as well as bacteriostatic against Mycobacterium ...
-
INDICATIONS & USAGEDermatitis herpetiformis: (D.H.) Leprosy: All forms of leprosy except for cases of proven Dapsone resistance.
-
CONTRAINDICATIONSHypersensitivity to Dapsone and/or its derivatives.
-
WARNINGSThe patient should be warned to respond to the presence of clinical signs such as sore throat, fever, pallor, purpura or jaundice. Deaths associated with the administration of Dapsone have been ...
-
PRECAUTIONSGeneral: Hemolysis and Heinz body formation may be exaggerated in individuals with a glucose-6-phosphate dehydrogenase (G6PD) deficiency, or methemoglobin reductase deficiency, or hemoglobin M ...
-
ADVERSE REACTIONSIn addition to the warnings listed above, the following syndromes and serious reactions have been reported in patients on Dapsone. Hematologic Effects: Dose-related hemolysis is the most common ...
-
OVERDOSAGENausea, vomiting, hyperexcitability can appear a few minutes up to 24 hours after ingestion of an overdosage. Methemoglobin induced depression, convulsions or severe cyanosis requires prompt ...
-
DOSAGE & ADMINISTRATIONDermatitis herpetiformis: The dosage should be individually titrated starting in adults with 50 mg daily and correspondingly smaller doses in children. If full control is not achieved within ...
-
LEPROSY REACTIONAL STATESAbrupt changes in clinical activity occur in leprosy with any effective treatment and are known as reactional states. The majority can be classified into two groups. The “Reversal” reaction ...
-
HOW SUPPLIEDDapsone Tablets USP, 25 mg are available as round, white to off-white, biconvex tablet debossed with C119 on one side and break line on other side. Bottle of 30 Tablets NDC ...
-
REFERENCES1. Lee, B., et al., Zidovudine, Trimethoprim, and Dapsone Pharmacokinetic Interactions in Patients with HIV Infection. Antimicrobial Agents and Chemotherapy , May 1996; 1231-1236. 2 ...
-
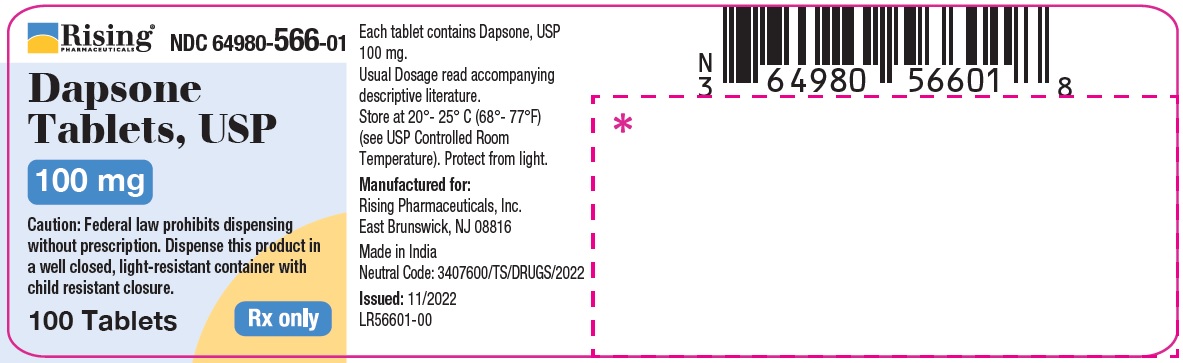
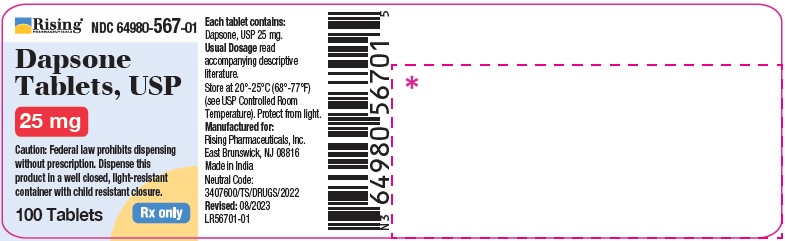
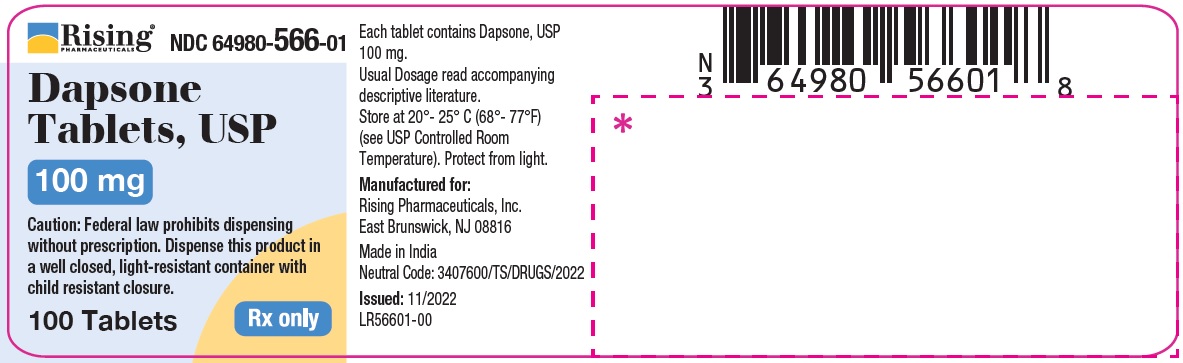
PACKAGE LABEL.PRINCIPAL DISPLAY PANELRising Pharmaceuticals, Inc. Dapsone Tablets, USP - NDC 64980-566-01 - 100 mg (100 Tablets) Rx only - Rising Pharmaceuticals, Inc. Dapsone Tablets, USP - NDC 64980-567-01 ...
-
INGREDIENTS AND APPEARANCEProduct Information