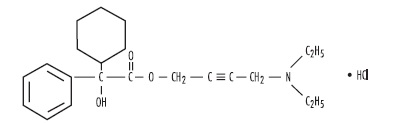Label: OXYBUTYNIN CHLORIDE tablet
- NDC Code(s): 64980-431-01, 64980-431-10, 64980-431-50, 64980-531-01
- Packager: RISING PHARMA HOLDINGS, INC.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 3, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTIONEach scored oxybutynin chloride tablet contains 2.5 mg and 5 mg of oxybutynin chloride. Chemically, oxybutynin chloride is d,l (racemic) 4-diethylamino-2-butynyl phenylcyclohexylglycolate ...
-
CLINICAL PHARMACOLOGY
Oxybutynin chloride exerts a direct antispasmodic effect on smooth muscle and inhibits the muscarinic action of acetylcholine on smooth muscle. Oxybutynin chloride exhibits only one fifth of the ...
-
INDICATIONS AND USAGEOxybutynin Chloride is indicated for the relief of symptoms of bladder instability associated with voiding in patients with uninhibited neurogenic or reflex neurogenic bladder (i.e., urgency ...
-
CONTRAINDICATIONSOxybutynin Chloride is contraindicated in patients with urinary retention, gastric retention and other severe decreased gastrointestinal motility conditions, uncontrolled narrow-angle glaucoma and ...
-
WARNINGSAngioedema of the face, lips, tongue and/or larynx has been reported with oxybutynin. In some cases, angioedema occurred after the first dose. Angioedema associated with upper airway swelling may ...
-
PRECAUTIONSCentral Nervous System Effects - Oxybutynin is associated with anticholinergic central nervous system (CNS) effects (See ADVERSE REACTIONS). A variety of CNS anticholinergic effects have been ...
-
ADVERSE REACTIONSThe safety and efficacy of oxybutynin chloride was evaluated in a total of 199 patients in three clinical trials. These participants were treated with oxybutynin chloride 5 to 20 mg/day for up to ...
-
OVERDOSAGETreatment should be symptomatic and supportive. Activated charcoal as well as a cathartic may be administered. Overdosage with oxybutynin chloride has been associated with anticholinergic ...
-
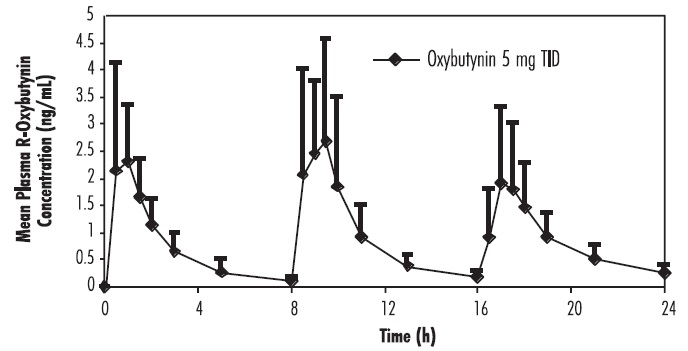
DOSAGE AND ADMINISTRATIONAdults - The usual dose is one 5-mg tablet two to three times a day. The maximum recommended dose is one 5-mg tablet four times a day. A lower starting dose of 2.5 mg two or three times a day ...
-
HOW SUPPLIEDOxybutynin Chloride Tablets USP, 2.5 mg are white to off-white round shaped tablets debossed “A2”on one side and plain on the other side. The tablets are available as follows: NDC Number ...
-
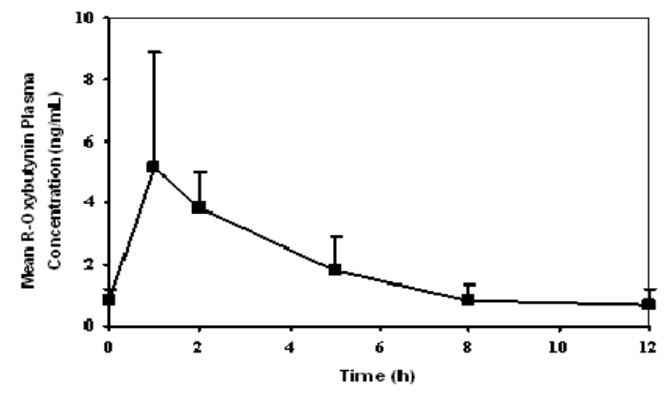
REFERENCES1. Yong C et al. Effect of Food on the Pharmacokinetics of Oxybutynin in normal subjects. Pharm Res. 1991; 8 (Suppl.): S-320. 2. Hughes KM et al. Measurement of oxybutynin and its N-desethyl ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELRising® NDC 64980-431-01 - Oxybutynin - Chloride - Tablets, USP - 5 mg - 100 Tablets Rx only - Rising® NDC 64980-431-50 - Oxybutynin ...
-
INGREDIENTS AND APPEARANCEProduct Information