Label: TOREMIFENE CITRATE tablet
- NDC Code(s): 64980-404-03
- Packager: Rising Pharma Holdings, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 5, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use TOREMIFENE CITRATE TABLETS safely and effectively. See full prescribing information for TOREMIFENE CITRATE TABLETS. TOREMIFENE ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: QT PROLONGATION
Toremifene citrate tablets have been shown to prolong the QTc interval in a dose- and concentration-related manner [see Clinical Pharmacology (12.2)]. Prolongation of the QT interval can result in a type of ventricular tachycardia called Torsade de pointes, which may result in syncope, seizure, and/or death. Toremifene should not be prescribed to patients with congenital/acquired QT prolongation, uncorrected hypokalemia or uncorrected hypomagnesemia. Drugs known to prolong the QT interval and strong CYP3A4 inhibitors should be avoided [see Warnings and Precautions (5.1)].
Close -
1 INDICATIONS AND USAGE
Toremifene citrate tablets are an estrogen agonist/antagonist indicated for the treatment of metastatic breast cancer in postmenopausal women with estrogen-receptor positive or unknown ...
-
2 DOSAGE AND ADMINISTRATION
The dosage of toremifene citrate tablets is 60 mg, once daily, orally. Treatment is generally continued until disease progression is observed.
-
3 DOSAGE FORMS AND STRENGTHS
Tablet is white to off-white, round, flat beveled edge tablet with “TO” on one side and plain on the reverse.
-
4 CONTRAINDICATIONS
4.1 Hypersensitivity to the Drug - Toremifene citrate tablets are contraindicated in patients with known hypersensitivity to the drug. 4.2 QT Prolongation, Hypokalemia ...
-
5 WARNINGS AND PRECAUTIONS
5.1 Prolongation of the QT Interval - Toremifene has been shown to prolong the QTc interval in a dose- and concentration-related manner [see Clinical Pharmacology (12.2)]. Prolongation of the QT ...
-
6 ADVERSE REACTIONS
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials ...
-
7 DRUG INTERACTIONS
7.1 Drugs that Decrease Renal Calcium Excretion - Drugs that decrease renal calcium excretion, e.g., thiazide diuretics, may increase the risk of hypercalcemia in patients receiving toremifene ...
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Based on its mechanism of action in humans and findings of increased pregnancy loss and fetal malformation in animal studies, toremifene citrate tablets can cause fetal harm when ...
-
10 OVERDOSAGE
Lethality was observed in rats following single oral doses that were ≥1000 mg/kg (about 150 times the recommended human dose on a mg/m2 basis) and was associated with gastric atony/dilatation ...
-
11 DESCRIPTION
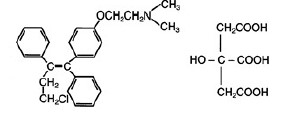
Toremifene citrate tablets for oral administration each contain 88.5 mg of toremifene citrate, which is equivalent to 60 mg toremifene. Toremifene citrate tablets are an estrogen ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - Toremifene is a nonsteroidal triphenylethylene derivative. Toremifene binds to estrogen receptors and may exert estrogenic, antiestrogenic, or both activities ...
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, and Impairment of Fertility - Conventional carcinogenesis studies in rats at doses of 0.12 to 12 mg/kg/day (approximately 1/50 to 2 times the daily maximum ...
-
14 CLINICAL STUDIES
Three prospective, randomized, controlled clinical studies (North American, Eastern European, and Nordic) were conducted to evaluate the efficacy of toremifene citrate tablets for the treatment of ...
-
16 SUPPLIED/STORAGE AND HANDLING
Toremifene citrate tablets, containing toremifene citrate in an amount equivalent to 60 mg of toremifene, are white to off-white, round, flat beveled edge tablet with “TO” on one side and plain on ...
-
17 PATIENT COUNSELING INFORMATION
Vaginal bleeding has been reported in patients using toremifene citrate tablets. Patients should be informed about this and instructed to contact their physician if such bleeding or other ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 64980-404-03 - Toremifene Citrate - Tablets - 30 TABLETS - Rx Only - 60 mg - Rising Pharma Holdings, Inc.
-
INGREDIENTS AND APPEARANCEProduct Information