Label: FAMCICLOVIR tablet, film coated
- NDC Code(s): 64980-349-03, 64980-350-03, 64980-351-03
- Packager: Rising Pharma Holdings, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use FAMCICLOVIR TABLETS safely and effectively. See full prescribing information for FAMCICLOVIR TABLETS. FAMCICLOVIR tablets, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Immunocompetent Adult Patients - Herpes labialis (cold sores): Famciclovir tablets are indicated for the treatment of recurrent herpes labialis in adult patients. Genital herpes ...
-
2 DOSAGE AND ADMINISTRATIONFamciclovir tablets may be taken with or without food. 2.1 Dosing Recommendation in Immunocompetent Adult Patients - Herpes labialis (cold sores): The recommended dosage of famciclovir ...
-
3 DOSAGE FORMS AND STRENGTHSFamciclovir tablets, USP are available in 3 strengths: 125 mg: White to pale yellow colored, round, film-coated, biconvex tablets with beveled edges, debossed with ‘X’ on one side and ‘48’ on ...
-
4 CONTRAINDICATIONSFamciclovir tablets are contraindicated in patients with known hypersensitivity to the product, its components, or Denavir® (penciclovir cream).
-
5 WARNINGS AND PRECAUTIONS5.1 Acute Renal Failure - Cases of acute renal failure have been reported in patients with underlying renal disease who have received inappropriately high doses of famciclovir for their level of ...
-
6 ADVERSE REACTIONSAcute renal failure is discussed in greater detail in other sections of the label [see Warnings and Precautions (5)]. The most common adverse events reported in at least 1 indication by ...
-
7 DRUG INTERACTIONS7.1 Potential for Famciclovir to Affect Other Drugs - The steady-state pharmacokinetics of digoxin were not altered by concomitant administration of multiple doses of famciclovir (500 mg three ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available data from pharmacovigilance reports with famciclovir use in pregnant women have not identified a drug-associated risk of major birth defects ...
-
10 OVERDOSAGEAppropriate symptomatic and supportive therapy should be given. Penciclovir is removed by hemodialysis.
-
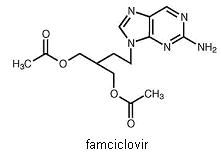
11 DESCRIPTIONThe active ingredient in famciclovir tablets, USP is famciclovir, an orally administered prodrug of the antiviral agent penciclovir. Chemically, famciclovir is known as ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Famciclovir is an orally administered prodrug of the anti-alpha herpes viral agent penciclovir [see Microbiology (12.4)]. 12.3 Pharmacokinetics - Famciclovir ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis: Two-year dietary carcinogenicity studies with famciclovir were conducted in rats and mice. An increase in the ...
-
14 CLINICAL STUDIES14.1 Herpes Labialis (Cold Sores) A randomized, double-blind, placebo-controlled trial was conducted in 701 immunocompetent adults with recurrent herpes labialis. Patients self-initiated ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGFamciclovir Tablets USP, 125 mg are white to pale yellow colored, round, film-coated, biconvex tablets with beveled edges, debossed with ‘X’ on one side and ‘48’ on the other ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). There is no evidence that famciclovir will affect the ability of a patient to drive or to use machines. However ...
-
PATIENT INFORMATIONFamciclovir Tablets, USP - (fam sye' kloe vir) Rx only - What are famciclovir tablets? Famciclovir tablets are a prescription antiviral medicine used: in adults with a normal immune ...
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 125 mg (30 Tablets Bottle)Rising® NDC 64980-349-03 - Famciclovir - Tablets, USP - 125 mg - 30 Tablets Rx only
-
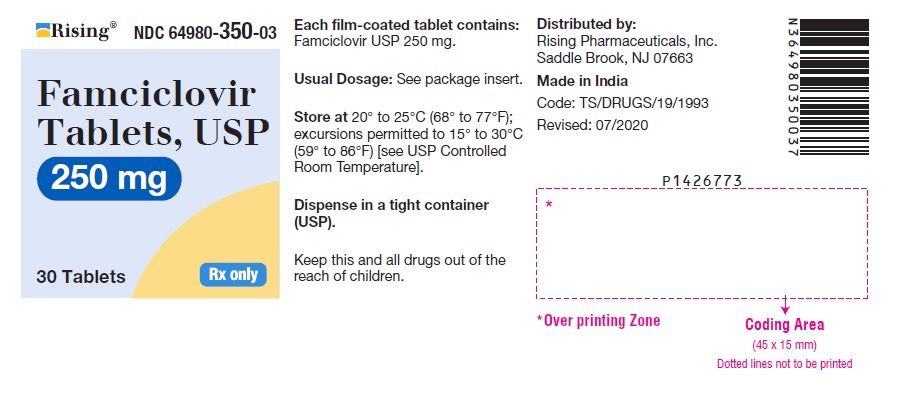
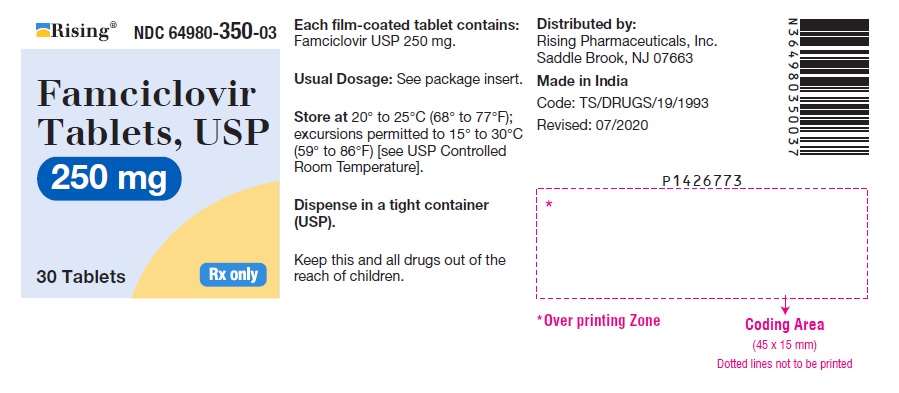
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 250 mg (30 Tablets Bottle)Rising® NDC 64980-350-03 - Famciclovir - Tablets, USP - 250 mg - 30 Tablets Rx only
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 500 mg (30 Tablets Bottle)Rising® NDC 64980-351-03 - Famciclovir - Tablets, USP - 500 mg - 30 Tablets Rx only
-
INGREDIENTS AND APPEARANCEProduct Information