Label: ERYTHROMYCIN AND BENZOYL PEROXIDE gel
- NDC Code(s): 64980-328-01, 64980-328-02
- Packager: Rising Pharma Holdings, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 23, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
TO THE PHARMACIST: IMPORTANT: PLEASE READ COMPLETE COMPOUNDING DIRECTIONS
NOTE: PRIOR TO DISPENSING, TAP VIAL UNTIL ALL POWDER FLOWS FREELY. ADD INDICATED AMOUNT OF ROOM TEMPERATURE 70% ETHYL ALCOHOL TO VIAL (TO THE MARK) AND IMMEDIATELY SHAKE VIGOROUSLY TO COMPLETELY ...
-
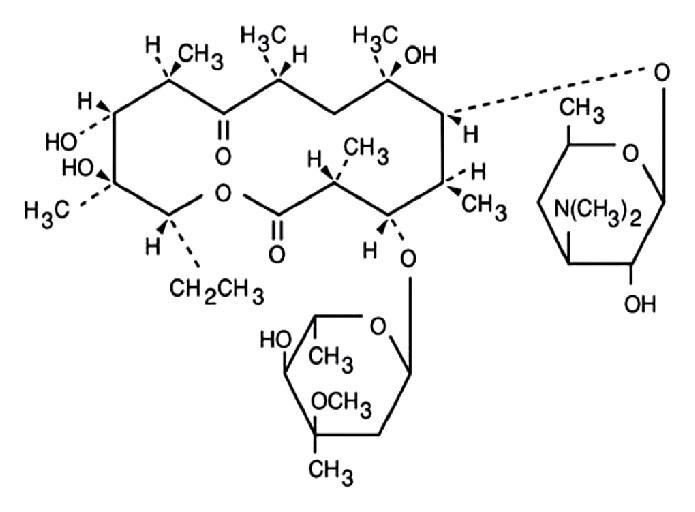
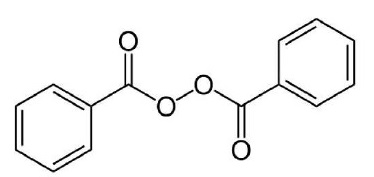
DESCRIPTIONErythromycin and Benzoyl Peroxide Topical Gel USP contains erythromycin [(3R*, 4S*, 5S*, 6R*, 7R*, 9R*, 11R*, 12R*, 13S* ...
-
CLINICAL PHARMACOLOGYThe exact mechanism by which erythromycin reduces lesions of acne vulgaris is not fully known; however, the effect appears to be due in part to the antibacterial activity of the drug. Benzoyl ...
-
INDICATIONS AND USAGEErythromycin and Benzoyl Peroxide Topical Gel USP is indicated for the topical treatment of acne vulgaris.
-
CONTRAINDICATIONSErythromycin and Benzoyl Peroxide Topical Gel USP is contraindicated in those individuals who have shown hypersensitivity to any of its components.
-
WARNINGSPseudomembranous colitis has been reported with nearly all antibacterial agents, including erythromycin, and may range in severity from mild to life-threatening. Therefore, it is important to ...
-
PRECAUTIONSGeneral: For topical use only; not for ophthalmic use. Concomitant topical acne therapy should be used with caution because a possible cumulative irritancy effect may occur, especially with ...
-
ADVERSE REACTIONSIn controlled clinical trials, the incidence of adverse reactions associated with the use of Erythromycin and Benzoyl Peroxide Topical Gel USP was approximately 3%. These were dryness and ...
-
DOSAGE AND ADMINISTRATIONErythromycin and Benzoyl Peroxide Topical Gel USP should be applied twice daily, morning and evening, or as directed by a physician, to affected areas after the skin is thoroughly washed, rinsed ...
-
PRINCIPAL DISPLAY PANEL———PRINCIPAL DISPLAY PANEL 23.3g——— Rising® NDC 64980-328-23 - Erythromycin and - Benzoyl Peroxide - Topical Gel USP - FOR TOPICAL USE ONLY ...
-
PRINCIPAL DISPLAY PANEL———PRINCIPAL DISPLAY PANEL 46.6g——— Rising® NDC 64980-328-46 - Erythromycin and - Benzoyl Peroxide - Topical Gel USP - FOR TOPICAL USE ONLY ...
-
INGREDIENTS AND APPEARANCEProduct Information