Label: DIPYRIDAMOLE tablet, film coated
- NDC Code(s): 64980-133-01, 64980-133-10, 64980-134-01, 64980-134-10, view more
- Packager: Rising Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 28, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONDipyridamole Tablets, USP - 25 mg, 50 mg, and 75 mg tablets - Rx only - Prescribing Information
-
DESCRIPTION
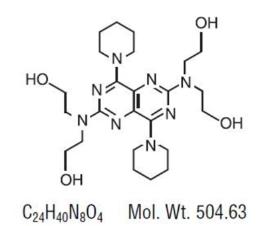
Dipyridamole USP is a platelet inhibitor chemically described as 2,2',2",2"'-[(4,8- Dipiperidinopyrimido[5,4-d]pyrimidine-2,6-diyl)dinitrilo]-tetraethanol. It has the following structural ...
-
CLINICAL PHARMACOLOGY
It is believed that platelet reactivity and interaction with prosthetic cardiac valve surfaces, resulting in abnormally shortened platelet survival time, is a significant factor in thromboembolic ...
-
INDICATIONS AND USAGE
Dipyridamole tablets are indicated as an adjunct to coumarin anticoagulants in the prevention of postoperative thromboembolic complications of cardiac valve replacement.
-
CONTRAINDICATIONS
Hypersensitivity to dipyridamole and any of the other components.
-
PRECAUTIONS
General - Coronary Artery Disease: Dipyridamole has a vasodilatory effect and should be used with caution in patients with severe coronary artery disease (e.g., unstable angina or recently ...
-
ADVERSE REACTIONS
Adverse reactions at therapeutic doses are usually minimal and transient. On long-term use of dipyridamole tablets initial side effects usually disappear. The following reactions in Table 1 were ...
-
OVERDOSAGE
In case of real or suspected overdose, seek medical attention or contact a Poison Control Center immediately. Careful medical management is essential. Based upon the known hemodynamic effects of ...
-
DOSAGE AND ADMINISTRATION
Adjunctive Use in Prophylaxis of Thromboembolism after Cardiac Valve Replacement. The recommended dose is 75 mg to 100 mg four times daily as an adjunct to the usual warfarin therapy. Please note ...
-
HOW SUPPLIED
Dipyridamole Tablets, USP are available as round, white, film-coated tablets of 25 mg, 50 mg, and 75 mg coded 81/SL, 82/SL, and 83/SL, respectively. They are available in bottles of 100 tablets as ...
-
PRINCIPAL DISPLAY PANEL
Container 25 mg - Rising NDC 64980-133-01 - Dipyridamole - Tablets, USP - 25 mg - 100 Tablets - Rx only
-
PRINCIPAL DISPLAY PANEL
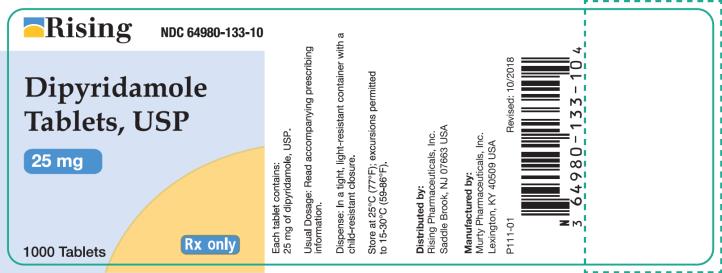
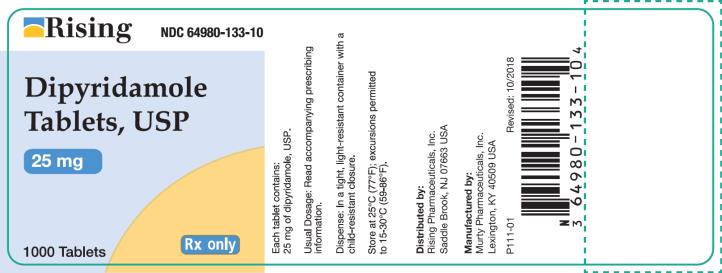
Container 25 mg - Rising NDC 64980-133-10 - Dipyridamole - Tablets, USP - 25 mg - 1000 Tablets - Rx only
-
PRINCIPAL DISPLAY PANEL
Container 50 mg - Rising NDC 64980-134-01 - Dipyridamole - Tablets, USP - 50 mg - 100 Tablets - Rx only
-
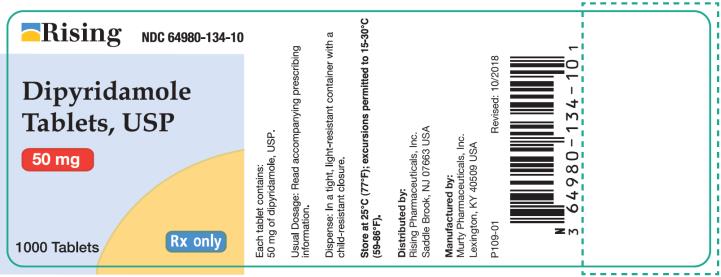
PRINCIPAL DISPLAY PANEL
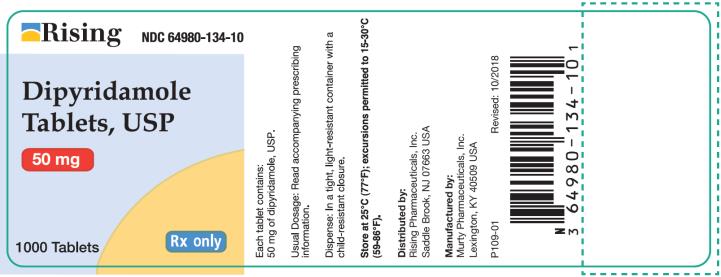
Container 50 mg - Rising NDC 64980-134-10 - Dipyridamole - Tablets, USP - 50 mg - 1000 Tablets - Rx only
-
PRINCIPAL DISPLAY PANEL
Container 75 mg - Rising NDC 64980-135-01 - Dipyridamole - Tablets, USP - 75 mg - 100 Tablets - Rx only
-
PRINCIPAL DISPLAY PANEL
Container 75 mg - Rising NDC 64980-135-10 - Dipyridamole - Tablets, USP - 75 mg - 1000 Tablets - Rx only
-
INGREDIENTS AND APPEARANCEProduct Information