Label: ULORIC- febuxostat tablet
- NDC Code(s): 64764-677-11, 64764-677-13, 64764-677-19, 64764-677-30, view more
- Packager: Takeda Pharmaceuticals America, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated April 27, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ULORIC safely and effectively. See full prescribing information for ULORIC. ULORIC (febuxostat) tablets, for oral use - Initial U.S ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: CARDIOVASCULAR DEATH
Gout patients with established cardiovascular (CV) disease treated with ULORIC had a higher rate of CV death compared to those treated with allopurinol in a CV outcomes study [see Warnings and Precautions (5.1)].
Consider the risks and benefits of ULORIC when deciding to prescribe or continue patients on ULORIC. ULORIC should only be used in patients who have an inadequate response to a maximally titrated dose of allopurinol, who are intolerant to allopurinol, or for whom treatment with allopurinol is not advisable [see Indications and Usage (1)].
Close -
1 INDICATIONS AND USAGEULORIC is a xanthine oxidase (XO) inhibitor indicated for the chronic management of hyperuricemia in adult patients with gout who have an inadequate response to a maximally titrated dose of ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage - The recommended ULORIC dosage is 40 mg or 80 mg once daily. The recommended starting dosage of ULORIC is 40 mg once daily. For patients who do not achieve a serum uric ...
-
3 DOSAGE FORMS AND STRENGTHS40 mg tablets, light green to green, round, debossed with "TAP" and "40" 80 mg tablets, light green to green, teardrop shaped, debossed with "TAP" and "80"
-
4 CONTRAINDICATIONSULORIC is contraindicated in patients being treated with azathioprine or mercaptopurine [see Drug Interactions (7)].
-
5 WARNINGS AND PRECAUTIONS5.1 Cardiovascular Death - In a cardiovascular (CV) outcome study, gout patients with established CV disease treated with ULORIC had a higher rate of CV death compared to those treated with ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions are described elsewhere in the prescribing information: Cardiovascular Death [see Warnings and Precautions (5.1)] Hepatic Effects [see Warnings and ...
-
7 DRUG INTERACTIONS7.1 Xanthine Oxidase Substrate Drugs - ULORIC is an XO inhibitor. Based on a drug interaction study in healthy patients, febuxostat altered the metabolism of theophylline (a substrate of XO) in ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Limited available data with ULORIC use in pregnant women are insufficient to inform a drug associated risk of adverse developmental outcomes. No adverse ...
-
10 OVERDOSAGEULORIC was studied in healthy patients in doses up to 300 mg daily for seven days without evidence of dose-limiting toxicities. No overdose of ULORIC was reported in clinical studies. Patients ...
-
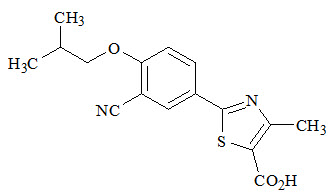
11 DESCRIPTIONULORIC (febuxostat) is a xanthine oxidase inhibitor. The active ingredient in ULORIC is 2-[3-cyano-4-(2-methylpropoxy) phenyl]-4-methylthiazole-5-carboxylic acid, with a molecular weight of ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - ULORIC, a xanthine oxidase inhibitor, achieves its therapeutic effect by decreasing serum uric acid. ULORIC is not expected to inhibit other enzymes involved in purine ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Two-year carcinogenicity studies were conducted in F344 rats and B6C3F1 mice. Increased transitional cell papilloma and carcinoma of ...
-
14 CLINICAL STUDIESA serum uric acid level of less than 6 mg/dL is the goal of antihyperuricemic therapy and has been established as appropriate for the treatment of gout. 14.1 Management of Hyperuricemia in ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGULORIC 40 mg tablets are light green to green in color, round, debossed with "TAP" on one side and "40" on the other side and supplied as: NDC NumberSize - 64764-918-11Hospital Unit Dose ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide). CV Death - Inform patients that gout patients with established CV disease treated with ULORIC had a higher rate ...
-
PATIENT PACKAGE INSERTThis Medication Guide has been approved by the U.S. Food and Drug AdministrationULR015 R10Revised: April 2023 MEDICATION GUIDE - ULORIC (Ū–'lor–ik) (febuxostat) tablets, for oral ...
-
PRINCIPAL DISPLAY PANEL - 40 mg Tablet Bottle LabelNDC 64764-918-30 - 30 Tablets - Uloric - (febuxostat) tablets - 40 mg - Dispense the accompanying - Medication Guide to each patient.
-
PRINCIPAL DISPLAY PANEL - 80 mg Tablet Bottle LabelNDC 64764-677-30 - 30 Tablets - Uloric - (febuxostat) tablets - 80 mg - Dispense the accompanying - Medication Guide to each patient.
-
INGREDIENTS AND APPEARANCEProduct Information