Label: URSODIOL tablet
- NDC Code(s): 64380-918-06, 64380-919-06
- Packager: Strides Pharma Science Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 13, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use URSODIOL TABLETS safely and effectively. See full prescribing information for URSODIOL TABLETS. URSODIOL tablets, for oral ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEUrsodiol tablets, 250 mg and 500 mg are indicated for the treatment of patients with primary biliary cholangitis (PBC).
-
2 DOSAGE AND ADMINISTRATION2.1 General Dosing Information - The recommended adult dosage for ursodiol tablets, 250 mg and 500 mg in the treatment of PBC is 13-15 mg/kg/day administered in two to four divided doses with ...
-
3 DOSAGE FORMS AND STRENGTHSUrsodiol: 250 mg tablet - Ursodiol: 500 mg scored tablet
-
4 CONTRAINDICATIONSPatients with complete biliary obstruction and known hypersensitivity or intolerance to ursodiol or any of the components of the formulation.
-
5 WARNINGS AND PRECAUTIONS5.1 Abnormal Liver Function Tests - Liver function tests (γ-GT, alkaline phosphatase, AST, ALT) and bilirubin levels should be monitored every month for three months after start of therapy, and ...
-
6 ADVERSE REACTIONS6.1 Clinical Studies Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONS7.1 Bile Acid Sequestering Agents - Bile acid sequestering agents such as cholestyramine and colestipol may interfere with the action of ursodiol by reducing its absorption. 7.2 ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available published data on the use of ursodiol in pregnant women derived from randomized controlled trials, observational studies, and case series collected over ...
-
10 OVERDOSAGEThere have been no reports of accidental or intentional overdosage with ursodiol. Single oral doses of ursodiol at 10 g/kg in mice and dogs, and 5 g/kg in rats were not lethal. A single oral dose ...
-
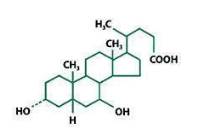
11 DESCRIPTIONUrsodiol tablets, USP 250 mg is available as a film-coated tablet for oral administration. Ursodiol tablets, USP 500 mg is available as a scored film-coated tablet for oral administration ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Ursodiol, a naturally occurring hydrophilic bile acid, derived from cholesterol, is present as a minor fraction of the total human bile acid pool. Oral administration ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - In two 24-month oral carcinogenicity studies in mice, ursodiol at doses up to 1,000 mg/kg/day (3,000 mg/m2/day) was not tumorigenic ...
-
14 CLINICAL STUDIES14.1 Efficacy of Ursodeoxycholic Acid Administered at 13 to 15 mg/kg/day in 3 or 4 Divided Doses to PBC Patients - A U.S., multicenter, randomized, double-blind, placebo-controlled study was ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 Ursodiol Tablets, USP 250 mg - Each ursodiol tablet USP, 250 mg white to off white, elliptical shaped, biconvex film-coated tablet, debossed with "250" on one side and plain on the other ...
-
17 PATIENT COUNSELING INFORMATIONEnteroliths in Patients with Risk for Intestinal Stenosis or Stasis - Advise patients or their caretaker(s) to notify their healthcare provider if they experience obstructive gastrointestinal ...
-
PRINCIPAL DISPLAY PANELNDC 64380-918-06 - Ursodiol Tablets, USP 250 mg - Strides Pharma Inc - 100 Tablets - Rx only - NDC 64380-919-06 - Ursodiol Tablets, USP 500 mg - Strides Pharma Inc - 100 Tablets - Rx only
-
INGREDIENTS AND APPEARANCEProduct Information