Label: CALCITRIOL- calcitriol capsules 0.25 mcg capsule
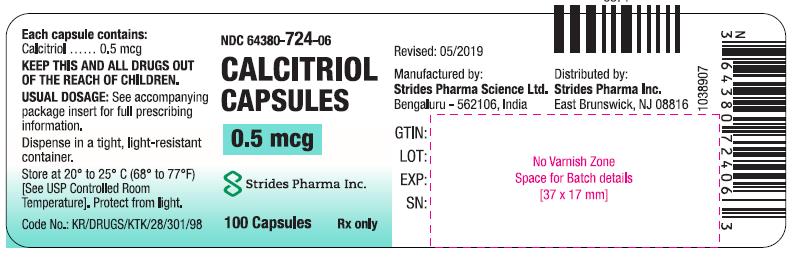
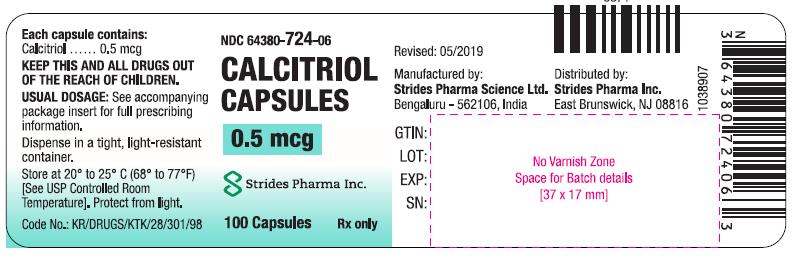
CALCITRIOL- calcitriol capsules 0.5 mcg capsule
- NDC Code(s): 64380-723-04, 64380-723-06, 64380-724-06
- Packager: Strides Pharma Science Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 23, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx only
-
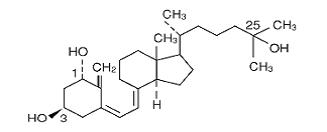
DESCRIPTIONCalcitriol is a synthetic vitamin D analog which is active in the regulation of the absorption of calcium from the gastrointestinal tract and its utilization in the body. Calcitriol is available ...
-
CLINICAL PHARMACOLOGYMan's natural supply of vitamin D depends mainly on exposure to the ultraviolet rays of the sun for conversion of 7-dehydrocholesterol in the skin to vitamin D3 (cholecalciferol). Vitamin D3 must ...
-
INDICATIONS AND USAGEPredialysis Patients - Calcitriol capsule is indicated in the management of secondary hyperparathyroidism and resultant metabolic bone disease in patients with moderate to severe chronic renal ...
-
CONTRAINDICATIONSCalcitriol should not be given to patients with hypercalcemia or evidence of vitamin D toxicity. Use of Calcitriol in patients with known hypersensitivity to Calcitriol (or drugs of the same ...
-
WARNINGSOverdosage of any form of vitamin D is dangerous (see OVERDOSAGE ). Progressive hypercalcemia due to overdosage of vitamin D and its metabolites may be so severe as to require emergency ...
-
PRECAUTIONSGeneral - Excessive dosage of calcitriol induces hypercalcemia and in some instances hypercalciuria; therefore, early in treatment during dosage adjustment, serum calcium should be determined ...
-
ADVERSE REACTIONSSince calcitriol is believed to be the active hormone which exerts vitamin D activity in the body, adverse effects are, in general, similar to those encountered with excessive vitamin D intake ...
-
OVERDOSAGEAdministration of calcitriol to patients in excess of their daily requirements can cause hypercalcemia, hypercalciuria, and hyperphosphatemia. Since calcitriol is a derivative of vitamin D, the ...
-
DOSAGE AND ADMINISTRATIONThe optimal daily dose of calcitriol capsules must be carefully determined for each patient. Calcitriol capsule can be administered orally as a capsule (0.25 mcg or 0.50 mcg). Calcitriol capsule ...
-
HOW SUPPLIEDCapsules: 0.25 mcg calcitriol in soft gelatin, orange, oval capsules, imprinted with 673; bottles of 30 (64380-723-04), and bottles of 100 (64380-723-06). Capsules: 0.5 mcg calcitriol in soft ...
-
SPL UNCLASSIFIED SECTIONCalcitriol Capsules should be protected from light. Store at 20° to 25°C (68° to 77°C F) [See USP Controlled Room Temperature]
-
REFERENCES1. Jones CL, et al. Comparisons between oral and intraperitoneal 1, 25-dihydroxyvitamin D3 therapy in children treated with peritoneal dialysis. Clin Nephrol. 1994; 42:44-49.
-
SPL UNCLASSIFIED SECTIONManufactured by: Strides Pharma Science Ltd. Bengaluru - 562106, India - Distributed by: Strides Pharma Inc. East Brunswick, NJ 08816 - Revised: 05/2019
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELCalcitriol Capsules 0.25 mcg (30 s) Calcitriol Capsules 0.25 mcg (100 s) Calcitriol Capsules 0.5 mcg (100 s)
-
INGREDIENTS AND APPEARANCEProduct Information