Label: ZILEUTON tablet, multilayer, extended release
- NDC Code(s): 64380-189-01
- Packager: Strides Pharma Science Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 29, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ZILEUTON EXTENDED-RELEASE TABLETS safely and effectively. See full prescribing information for ZILEUTON EXTENDED-RELEASE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEZileuton extended-release tablets are indicated for the prophylaxis and chronic treatment of asthma in adults and children 12 years of age and older. Zileuton extended-release tablets are not ...
-
2 DOSAGE AND ADMINISTRATIONThe recommended dosage of Zileuton extended-release tablets for the treatment of patients with asthma is two 600 mg extended-release tablets twice daily, within one hour after morning and evening ...
-
3 DOSAGE FORMS AND STRENGTHSExtended-release tablets, 600 mg.
-
4 CONTRAINDICATIONSThe use of Zileuton extended-release tablets is contraindicated in patients with: Active liver disease or persistent hepatic function enzyme elevations greater than or equal to 3 times the ...
-
5 WARNINGS AND PRECAUTIONS5.1 Hepatotoxicity - Elevations of one or more hepatic function enzymes and bilirubin may occur during zileuton extended-release tablets therapy. These laboratory abnormalities may progress to ...
-
6 ADVERSE REACTIONSHepatotoxicity: Elevations of one or more hepatic function enzymes and bilirubin may occur during zileuton extended-release tablets therapy [see Warnings and Precautions (5)]. The most commonly ...
-
7 DRUG INTERACTIONSThe following study results were obtained using zileuton immediate-release tablets but the conclusions also apply to zileuton extended-release tablets. 7.1 Theophylline - In a drug-interaction ...
-
8 USE IN SPECIFIC POPULATIONSInformation on specific populations is based on studies conducted with zileuton immediate-release tablets and is applicable to zileuton extended-release tablets. 8.1 Pregnancy - Risk Summary ...
-
10 OVERDOSAGEHuman experience of acute overdose with zileuton is limited. A patient in a clinical study took between 6.6 and 9.0 grams of zileuton immediate-release tablets in a single dose. Vomiting was ...
-
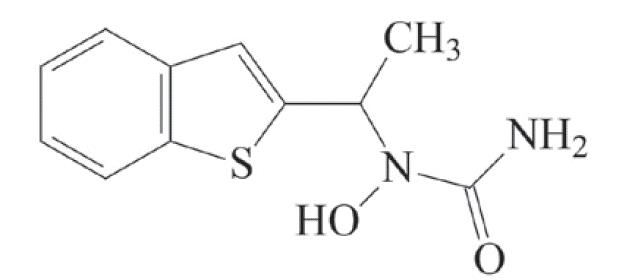
11 DESCRIPTIONZileuton is an orally active inhibitor of 5-lipoxygenase, the enzyme that catalyzes the formation of leukotrienes from arachidonic acid. Zileuton has the chemical name ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Zileuton is an inhibitor of 5-lipoxygenase and thus inhibits leukotriene (LTB4, LTC4, LTD4 and LTE4) formation. Both the R(+) and S(-) enantiomers are ...
-
13 NONCLINICAL TOXICOLOGY13.3 Carcinogenesis, Mutagenesis, Impairment of Fertility - In 2-year carcinogenicity studies, increases in the incidence of liver, kidney, and vascular tumors in female mice and a trend toward ...
-
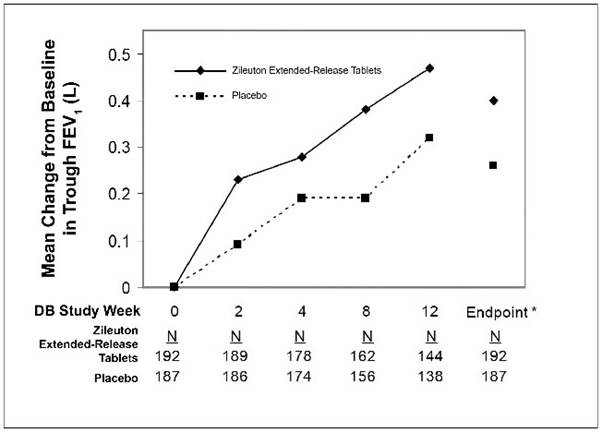
14 CLINICAL STUDIESThe efficacy of zileuton extended-release tablets was evaluated in a randomized, double-blind, parallel-group, placebo-controlled, multicenter trial of 12 weeks duration in patients 12 years of ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGZileuton Extended-Release Tablets, 600 mg are oblong, film-coated tablets with one red layer between two white layers, debossed on one side with "P723" and plain on other side; they are available ...
-
17 PATIENT COUNSELING INFORMATION17.1 Information for Patients - Patients should be told that: Zileuton extended-release tablets are indicated for the chronic treatment of asthma and should be taken regularly as prescribed ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCEProduct Information