Label: SODIUM SULFATE, POTASSIUM SULFATE AND MAGNESIUM SULFATE ORAL solution
- NDC Code(s): 64380-116-01, 64380-116-02
- Packager: Strides Pharma Science Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 12, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use, SODIUM SULFATE, POTASSIUM SULFATE and MAGNESIUM SULFATE ORAL SOLUTION safely and effectively. See full prescribing information for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGESodium sulfate, potassium sulfate and magnesium sulfate oral solution is indicated for cleansing of the colon as a preparation for colonoscopy in adult and pediatric patients 12 years of age and ...
-
2 DOSAGE AND ADMINISTRATION2.1 Dosage and Adminstration Overview - Administration of two bottles of sodium sulfate, potassium sulfate and magnesium sulfate oral solution and additional water is required for a complete ...
-
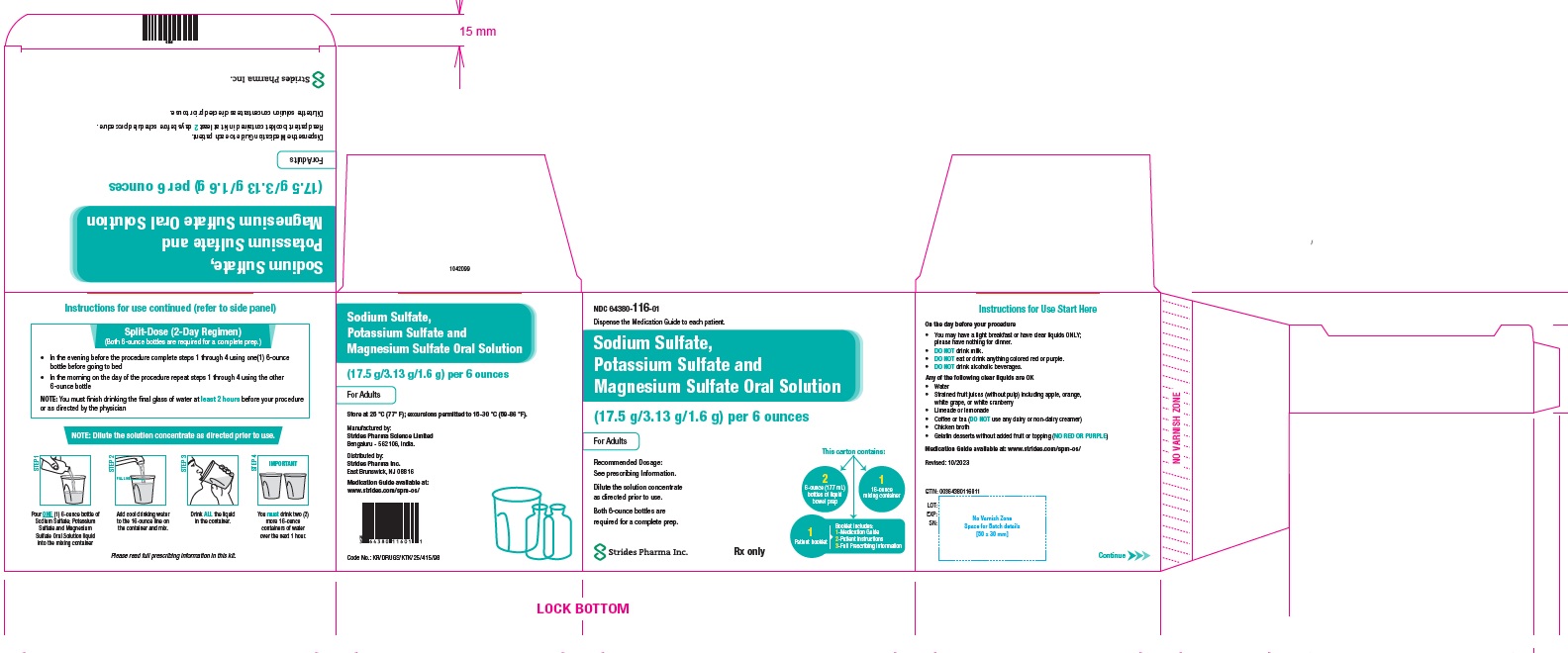
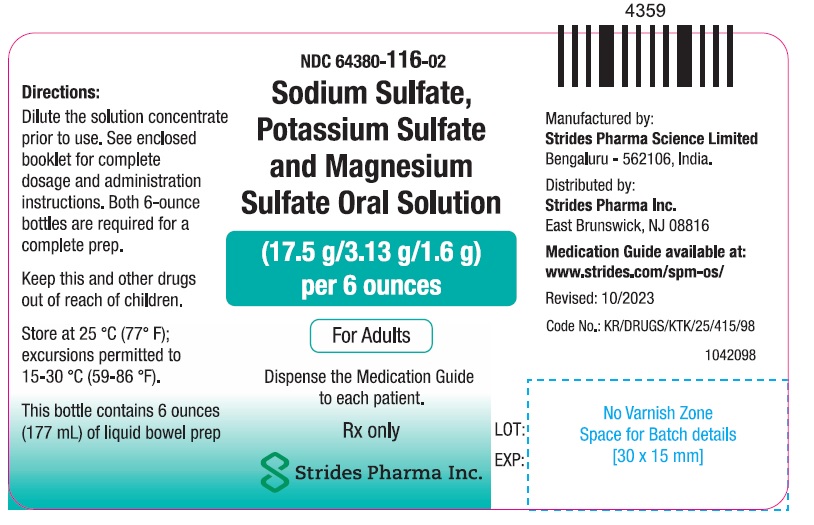
3 DOSAGE FORMS AND STRENGTHSSodium sulfate, potassium sulfate and magnesium sulfate oral solution (for adults): Two bottles each containing 6 ounces of an oral solution of 17.5 grams sodium sulfate, 3.13 grams potassium ...
-
4 CONTRAINDICATIONSSodium sulfate, potassium sulfate and magnesium sulfate oral solution is contraindicated in the following conditions: Gastrointestinal obstruction or ileus [see Warnings and Precautions ...
-
5 WARNINGS AND PRECAUTIONS5.1 Serious Fluid and Serum Chemistry Abnormalities - Advise all patients to hydrate adequately before, during, and after the use of sodium sulfate, potassium sulfate and magnesium sulfate oral ...
-
6 ADVERSE REACTIONSThe following important adverse reactions for bowel preparations are described elsewhere in the labeling: Serious Fluid and Serum Chemistry Abnormalities [see Warnings and Precautions ...
-
7 DRUG INTERACTIONS7.1 Drugs That May Increase Risks of Fluid and Electrolyte Abnormalities - Use caution when prescribing sodium sulfate, potassium sulfate and magnesium sulfate oral solution to patients taking ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no available data on sodium sulfate, potassium sulfate and magnesium sulfate oral solution use in pregnant women to evaluate for a drug associated risk ...
-
10 OVERDOSAGEOverdosage of more than the recommended dose of sodium sulfate, potassium sulfate and magnesium sulfate oral solution may lead to severe electrolyte disturbances, as well as dehydration and ...
-
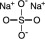
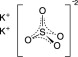
11 DESCRIPTIONSodium sulfate, potassium sulfate and magnesium sulfate oral solution (for adults) is an osmotic laxative and is provided as two bottles each containing 6 ounces of solution. Each bottle ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Sulfate salts provide sulfate anions, which are poorly absorbed. The osmotic effect of unabsorbed sulfate anions and the associated cations causes water to be retained ...
-
13 NONCLINICAL TOXICOLOGY13.2 Animal Toxicology and/or Pharmacology - The sulfate salts of sodium, potassium, and magnesium contained in sodium sulfate, potassium sulfate and magnesium sulfate oral solution were ...
-
14 CLINICAL STUDIESAdults - The colon cleansing efficacy of sodium sulfate, potassium sulfate and magnesium sulfate oral solution was evaluated in a randomized, single- blind, active-controlled, multicenter study in ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGEach sodium sulfate, potassium sulfate and magnesium sulfate oral solution (for adults) (NDC 64380-116-01) contains: Two bottles (NDC 64380-116-02) each containing 6-ounces of an oral solution ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient and/or caregiver to read the FDA-approved patient labeling (Medication Guide). Instruct patients or caregivers: Must dilute sodium sulfate, potassium sulfate and ...
-
MEDICATION GUIDEMEDICATION GUIDE - Sodium Sulfate (soe' dee um sul' fate), Potassium Sulfate (poe tas ' ee um sul' fate) and Magnesium Sulfate ( mag nee' zee um sul' fate) Oral Solution ...
-
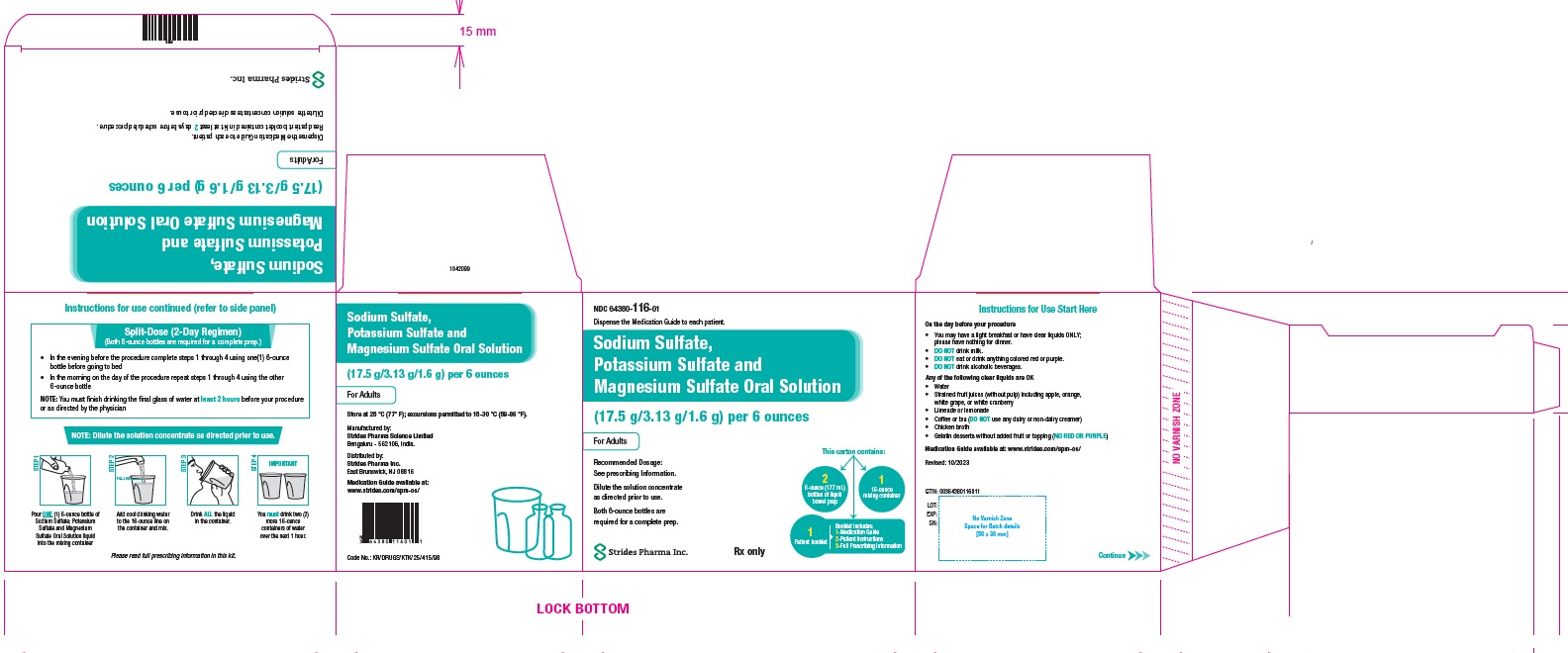
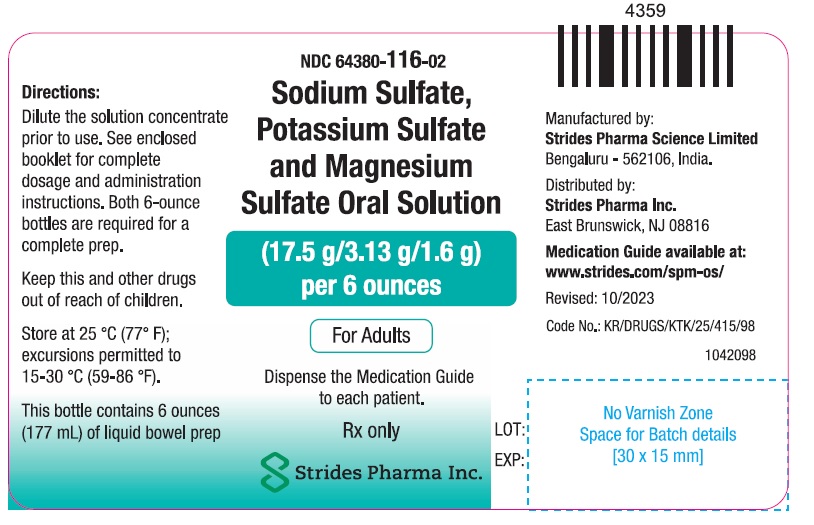
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC: 64380-116-01 - 177 ml Bottle Pack - Sodium Sulfate, Potassium Sulfate and Magnesium Sulfate Oral Solution (17.5 g/3.13 g/1.6 g) per 6 ounces - Dispense the enclosed Medication Guide to each ...
-
INGREDIENTS AND APPEARANCEProduct Information