Label: CIPROFLOXACIN AND DEXAMETHASONE suspension/ drops
- NDC Code(s): 62756-427-90
- Packager: Sun Pharmaceutical Industries, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 1, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use CIPROFLOXACIN AND DEXAMETHASONE OTIC SUSPENSION safely and effectively. See full prescribing information for CIPROFLOXACIN AND ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGECiprofloxacin and dexamethasone otic suspension USP is indicated for the treatment of infections caused by susceptible isolates of the designated microorganisms in the specific conditions listed ...
-
2 DOSAGE AND ADMINISTRATION2.1 Important Administration Instructions - Ciprofloxacin and dexamethasone otic suspension is for otic use (ears) only, and not for ophthalmic use, or for injection. Shake well immediately ...
-
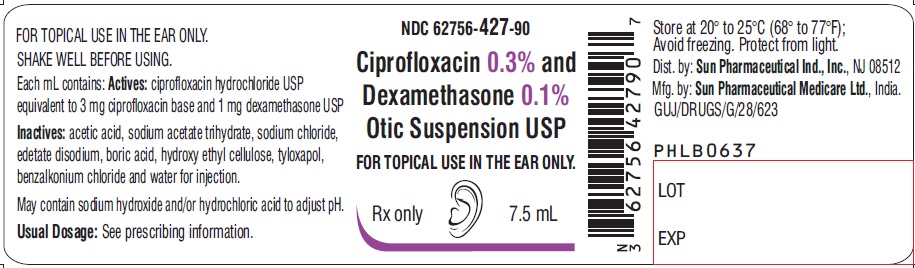
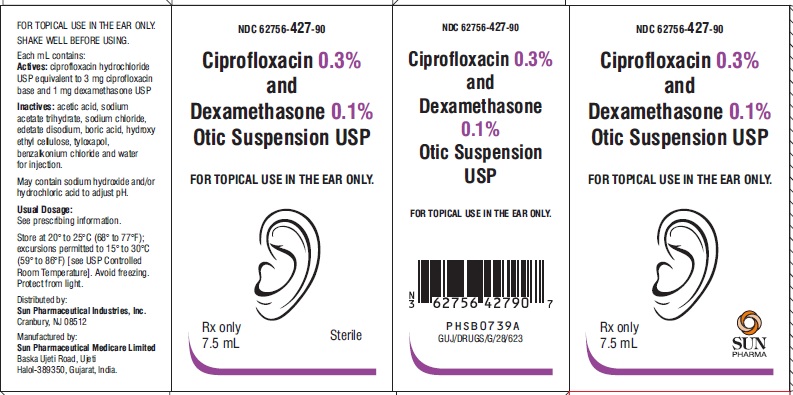
3 DOSAGE FORMS AND STRENGTHSOtic Suspension: Each mL of ciprofloxacin and dexamethasone otic suspension USP contains ciprofloxacin hydrochloride, USP 0.3 % (equivalent to 3 mg ciprofloxacin base) and dexamethasone, USP 0.1 ...
-
4 CONTRAINDICATIONSCiprofloxacin and dexamethasone otic suspension is contraindicated in patients with a history of hypersensitivity to ciprofloxacin, to other quinolones, or to any of the components in this ...
-
5 WARNINGS AND PRECAUTIONS5.1 Hypersensitivity Reactions - Ciprofloxacin and dexamethasone otic suspension should be discontinued at the first appearance of a skin rash or any other sign of hypersensitivity. Serious and ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions are described elsewhere in the labeling: Hypersensitivity Reactions [see Warnings and Precautions (5.1)] Potential for Microbial Overgrowth with ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no available data on ciprofloxacin and dexamethasone otic suspension use in pregnant women to evaluate for a drug-associated risk of major birth defects ...
-
10 OVERDOSAGEDue to the characteristics of this preparation, no toxic effects are to be expected with an otic overdose of this product.
-
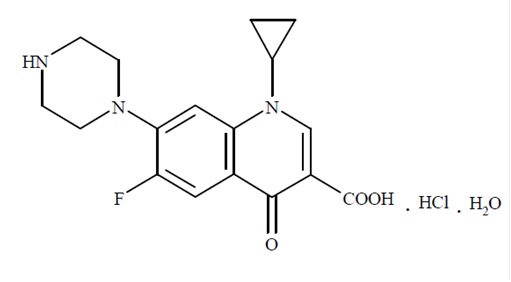
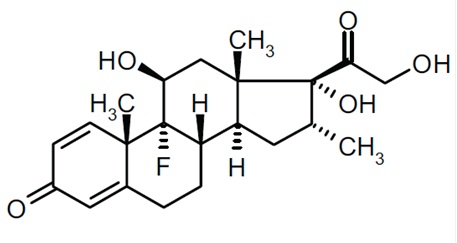
11 DESCRIPTIONCiprofloxacin and dexamethasone (ciprofloxacin 0.3% and dexamethasone 0.1%) sterile otic suspension contains the quinolone antimicrobial, ciprofloxacin hydrochloride, combined with the ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Ciprofloxacin is a fluoroquinolone antibacterial [see Microbiology (12.4)]. Dexamethasone, a corticosteroid, has been shown to suppress inflammation by inhibiting ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment Of Fertility - Carcinogenesis - Long-term carcinogenicity studies in mice and rats have been completed for ciprofloxacin. After daily oral doses of ...
-
14 CLINICAL STUDIESIn 2 randomized multicenter, controlled clinical trials, ciprofloxacin and dexamethasone otic suspension dosed 2 times per day for 7 days demonstrated clinical cures in 87% and 94% of per protocol ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied: Ciprofloxacin and dexamethasone (ciprofloxacin 0.3% and dexamethasone 0.1%) otic suspension USP is a white-to off-white suspension supplied as follows: 7.5 mL fill in a natural low ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use). For Otic Use Only - Advise patients that ciprofloxacin and dexamethasone otic ...
-
PATIENT INFORMATIONPATIENT INFORMATION - Ciprofloxacin (SIP-roe-FLOX-a-sin) and Dexamethasone (dex-a-meth-a-sone) Otic Suspension USP - What is ciprofloxacin and dexamethasone otic suspension ...
-
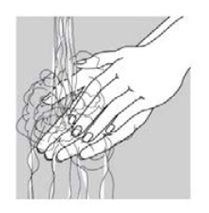
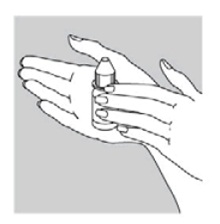
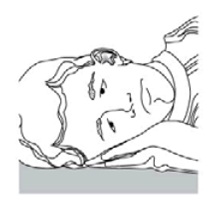
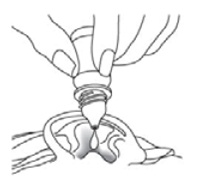
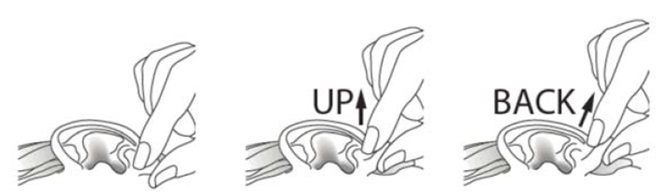
INSTRUCTIONS FOR USEINSTRUCTIONS FOR USE - Ciprofloxacin (SIP-roe-FLOX-a-sin) and Dexamethasone (dex-a-meth-a-sone) Otic Suspension USP - This “Instructions for Use” contains information on how to use ...
-
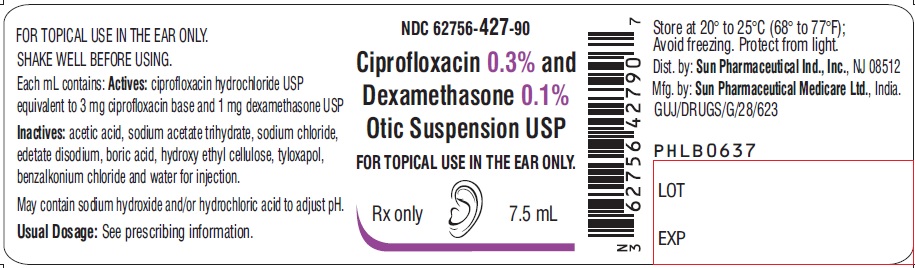
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL-LabelNDC 62756-427-90 - Ciprofloxacin 0.3% and Dexamethasone 0.1% Otic Suspension USP - FOR TOPICAL USE IN THE EAR ONLY. Rx only - 7.5 mL
-
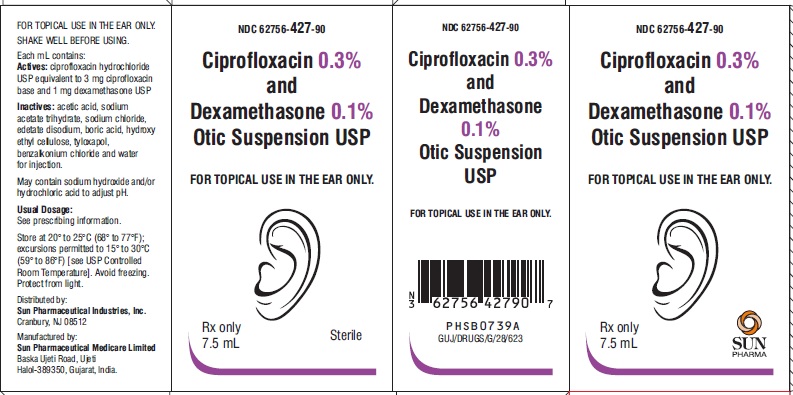
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL-ShowboxNDC 62756-427-90 - Ciprofloxacin 0.3% and Dexamethasone 0.1% Otic Suspension USP - FOR TOPICAL USE IN THE EAR ONLY. Rx only - 7.5 mL - Sterile
-
INGREDIENTS AND APPEARANCEProduct Information