Label: CEPHALEXIN capsule
-
Contains inactivated NDC Code(s)
NDC Code(s): 62756-293-13, 62756-293-88, 62756-294-13, 62756-294-88 - Packager: Sun Pharmaceutical Industries Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 14, 2010
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
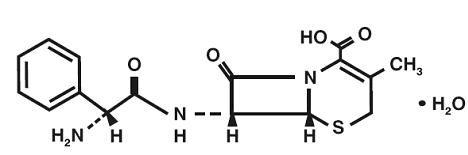
DESCRIPTIONCephalexin Capsules, USP is a semisynthetic cephalosporin antibiotic intended for oral administration. It is 7-(D-a-Amino-a-phenylacetamido)-3-methyl-3-cephem-4-carboxylic acid monohydrate ...
-
CLINICAL PHARMACOLOGYHuman Pharmacology - Cephalexin Capsule, USP is acid stable and may be given without regard to meals. It is rapidly absorbed after oral administration. Following doses of 250 mg, 500 mg, and ...
-
INDICATIONS AND USAGECephalexin capsules, USP are indicated for the treatment of the following infections when caused by susceptible strains of the designated microorganisms: Respiratory tract infections ...
-
CONTRAINDICATIONSCephalexin capsules, USP are contraindicated in patients with known allergy to the cephalosporin group of antibiotics.
-
WARNINGSBEFORE THERAPY WITH CEPHALEXIN IS INSTITUTED, CAREFUL INQUIRY SHOULD BE MADE TO DETERMINE WHETHER THE PATIENT HAS HAD PREVIOUS HYPERSENSITIVITY REACTIONS TO CEPHALEXIN, CEPHALOSPORINS ...
-
PRECAUTIONSGeneral - Prescribing cephalexin capsules, USP in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and ...
-
ADVERSE REACTIONSGastrointestinal — Onset of pseudomembranous colitis may occur during or after antibacterial treatment. (See WARNINGS.) Nausea and vomiting have been reported rarely. The most frequent ...
-
OVERDOSAGESigns and Symptoms — Symptoms of oral overdose may include nausea, vomiting, epigastric distress, diarrhea, and hematuria. If other symptoms are present, it is probably secondary to an ...
-
DOSAGE AND ADMINISTRATIONCephalexin capsules, USP are administered orally. Adults — The adult dosage ranges from 1 to 4 g daily in divided doses. The usual adult dose is 250 mg every 6 hours. For the following ...
-
HOW SUPPLIEDCephalexin capsules, USP, are available in: The 250 mg cephalexin capsules, USP are a white to off white powder filled into size 2 capsules (opaque dark green cap and opaque white body) that ...
-
REFERENCESNational Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically — Fourth Edition. Approved Standard NCCLS Document ...
-
SPL UNCLASSIFIED SECTIONDistributed by: Caraco Pharmaceutical Laboratories, Ltd. 1150 Elijah McCoy Drive, Detroit, MI 48202 - Manufactured by: Sun Pharmaceutical Industries Ltd. Acme Plaza, Andheri-Kurla Road, Andheri ...
-
PRINCIPAL DISPLAY PANELPACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL - 250 MG - NDC 62756-293-88 - Cephalexin Capsules, USP - 250 mg - Rx only - 100 CAPSULES - SUN PHARMACEUTICAL INDUSTRIES LTD. PACKAGE ...
-
INGREDIENTS AND APPEARANCEProduct Information