Label: ARFORMOTEROL TARTRATE solution
- NDC Code(s): 62756-277-02, 62756-277-03
- Packager: Sun Pharmaceutical Industries, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 27, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ARFORMOTEROL TARTRATE INHALATION SOLUTION safely and effectively. See full prescribing information for ARFORMOTEROL TARTRATE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Maintenance Treatment of COPD - Arformoterol tartrate inhalation solution is indicated for the long-term, twice daily (morning and evening) maintenance treatment of bronchoconstriction in ...
-
2 DOSAGE AND ADMINISTRATIONThe recommended dose of arformoterol tartrate inhalation solution is one 15 mcg unit-dose vial administered twice daily (morning and evening) by nebulization. A total daily dose of greater than 30 ...
-
3 DOSAGE FORMS AND STRENGTHSArformoterol tartrate inhalation solution is supplied as a sterile solution for nebulization in low-density polyethylene unit-dose vials. Each 2 mL vial contains 15 mcg of arformoterol equivalent ...
-
4 CONTRAINDICATIONSArformoterol tartrate inhalation solution is contraindicated in patients with a history of hypersensitivity to arformoterol, racemic formoterol or to any other components of this product. Use of a ...
-
5 WARNINGS AND PRECAUTIONS5.1 Serious Asthma-Related Events - Hospitalizations, Intubations Deaths - The safety and efficacy of arformoterol tartrate inhalation solution in patients with asthma have not been established ...
-
6 ADVERSE REACTIONSLong-acting beta2-adrenergic agonists, such as arformoterol tartrate, as monotherapy (without inhaled corticosteroids) for asthma increase the risk of asthma-related events. Arformoterol tartrate ...
-
7 DRUG INTERACTIONS7.1 Adrenergic Drugs - If additional adrenergic drugs are to be administered by any route, they should be used with caution because the sympathetic effects of arformoterol may be potentiated [see ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no adequate and well-controlled studies in pregnant women. Arformoterol tartrate should only be used during pregnancy if the expected benefit to the ...
-
9 DRUG ABUSE AND DEPENDENCEThere were no reported cases of abuse or evidence of drug dependence with the use of arformoterol tartrate inhalation solution in the clinical trials.
-
10 OVERDOSAGEThe expected signs and symptoms associated with overdosage of arformoterol tartrate inhalation solution are those of excessive beta-adrenergic stimulation and/or occurrence or exaggeration of any ...
-
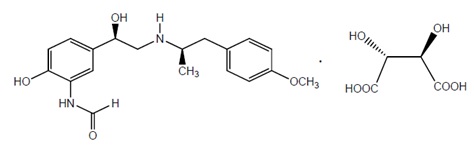
11 DESCRIPTIONArformoterol tartrate inhalation solution is a sterile, clear, colorless, aqueous solution of the tartrate salt of arformoterol, the (R,R)-enantiomer of formoterol. Arformoterol is a selective ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Arformoterol, the (R,R)-enantiomer of formoterol, is a selective long-acting beta2-adrenergic receptor agonist (beta2-agonist) that has two-fold greater potency ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term studies were conducted in mice using oral administration and rats using inhalation administration to evaluate the ...
-
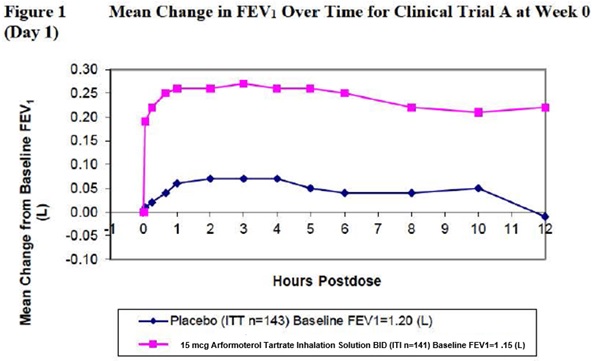
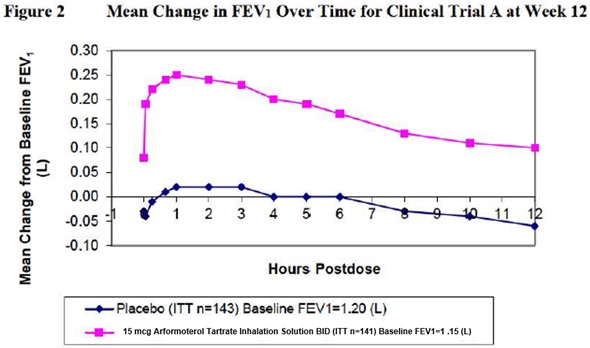
14 CLINICAL STUDIES14.1 Adult COPD Trials - Arformoterol tartrate inhalation solution was studied in two identical, 12-week, double-blind, placebo-and active-controlled, randomized, multi-center, parallel group ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGArformoterol tartrate inhalation solution is supplied in a single strength (15 mcg of arformoterol, equivalent to 22 mcg of arformoterol tartrate) as 2 mL of a sterile, clear, colorless aqueous ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use) with each new prescription and refill. The complete text of the Patient Information ...
-
Patient InformationDispense with Patient Information available at: https://www.sunpharma.com/usa/products - PATIENT INFORMATION - Arformoterol Tartrate (ar for MOE ter ol TAR-trate) Inhalation ...
-
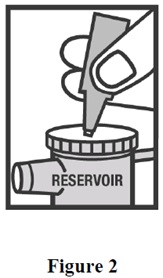
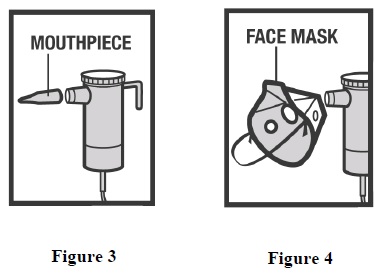
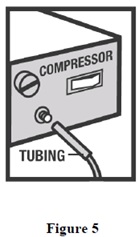
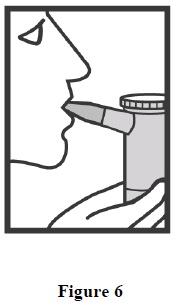
INSTRUCTIONS FOR USEInstructions for Using Arformoterol Tartrate Inhalation Solution - Arformoterol tartrate inhalation solution is used only in a standard jet nebulizer machine connected to an air compressor. Make ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - Pouch LabelNDC 62756-277-01 - Arformoterol Tartrate Inhalation Solution - 15 mcg*/2 mL - *Potency expressed as arformoterol - For Oral Inhalation Only - After opening the foil pouch, the unused vials should be ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - Carton LabelNDC 62756-277-02 - Arformoterol Tartrate Inhalation Solution - 15 mcg*/2 mL - *Potency expressed as arformoterol - For Oral Inhalation Only - KEEP REFRIGERATED OR STORE AT ROOM TEMPERATURE FOR UP TO 6 ...
-
INGREDIENTS AND APPEARANCEProduct Information