Label: ZONISAMIDE capsule
-
NDC Code(s):
62756-258-01,
62756-258-02,
62756-258-03,
62756-258-04, view more62756-259-01, 62756-259-02, 62756-259-03, 62756-259-04, 62756-260-01, 62756-260-02, 62756-260-03, 62756-260-04, 62756-260-05
- Packager: Sun Pharmaceutical Industries, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 24, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
DESCRIPTIONZonisamide is an antiseizure drug chemically classified as a sulfonamide and unrelated to other antiseizure agents. The active ingredient is zonisamide, 1,2-benzisoxazole-3-methanesulfonamide. The ...
Zonisamide is an antiseizure drug chemically classified as a sulfonamide and unrelated to other antiseizure agents. The active ingredient is zonisamide, 1,2-benzisoxazole-3-methanesulfonamide. The molecular formula is C8H8N2O3S with a molecular weight of 212.23. Zonisamide is a white powder, pKa = 10.2, and is moderately soluble in water (0.8 mg/mL) and 0.1 N HCl (0.5 mg/mL).
The chemical structure is:
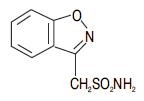
Zonisamide is supplied for oral administration as capsules containing 25 mg, 50 mg or 100 mg zonisamide, USP. Each capsule contains the labeled amount of zonisamide plus the following inactive ingredients: microcrystalline cellulose, hydrogenated vegetable oil, gelatin, and titanium dioxide.
In addition, individual empty hard gelatin capsule shell contains:
50 mg : Black iron oxide.
100 mg : FD&C Blue #1 and FD&C Red #40.
The imprinting ink contains black iron oxide, shellac glaze, propylene glycol and also contains either FD & C Blue No. 2, FD & C Red No. 40, FD & C Blue No. 1 and D & C Yellow No.10 or strong ammonia solution and potassium hydroxide.
Close -
CLINICAL PHARMACOLOGYMechanism of Action: The precise mechanism(s) by which zonisamide exerts its antiseizure effect is unknown. Zonisamide demonstrated anticonvulsant activity in several experimental models. In ...
Mechanism of Action: The precise mechanism(s) by which zonisamide exerts its antiseizure effect is unknown. Zonisamide demonstrated anticonvulsant activity in several experimental models. In animals, zonisamide was effective against tonic extension seizures induced by maximal electroshock but ineffective against clonic seizures induced by subcutaneous pentylenetetrazol. Zonisamide raised the threshold for generalized seizures in the kindled rat model and reduced the duration of cortical focal seizures induced by electrical stimulation of the visual cortex in cats. Furthermore, zonisamide suppressed both interictal spikes and the secondarily generalized seizures produced by cortical application of tungstic acid gel in rats or by cortical freezing in cats. The relevance of these models to human epilepsy is unknown.
Zonisamide may produce these effects through action at sodium and calcium channels. In vitro pharmacological studies suggest that zonisamide blocks sodium channels and reduces voltage-dependent, transient inward currents (T-type Ca2+ currents), consequently stabilizing neuronal membranes and suppressing neuronal hypersynchronization. In vitro binding studies have demonstrated that zonisamide binds to the GABA/benzodiazepine receptor ionophore complex in an allosteric fashion which does not produce changes in chloride flux. Other in vitro studies have demonstrated that zonisamide (10 to 30 mcg/mL) suppresses synaptically-driven electrical activity without affecting postsynaptic GABA or glutamate responses (cultured mouse spinal cord neurons) or neuronal or glial uptake of [3H]-GABA (rat hippocampal slices). Thus, zonisamide does not appear to potentiate the synaptic activity of GABA. In vivo microdialysis studies demonstrated that zonisamide facilitates both dopaminergic and serotonergic neurotransmission.
Zonisamide is a carbonic anhydrase inhibitor. The contribution of this pharmacological action to the therapeutic effects of zonisamide is unknown. However, as a carbonic anhydrase inhibitor, zonisamide may cause metabolic acidosis (see WARNINGS, Metabolic Acidosis subsection).
Pharmacokinetics:
Absorption
Following a 200 to 400 mg oral zonisamide dose, peak plasma concentrations (range: 2 to 5 mcg/mL) in normal volunteers occur within 2 to 6 hours. In the presence of food, the time to maximum concentration is delayed; occurring at 4 to 6 hours, but food has no effect on the bioavailability of zonisamide. Zonisamide absorption is dose-proportional in the range of 200 to 400 mg. Cmax and AUC, however, increase disproportionately at 800 mg, possibly due to saturable binding of zonisamide to red blood cells. Once a stable dose is reached, steady state is achieved within 14 days.
Distribution
The apparent volume of distribution (V/F) of zonisamide is about 1.45 L/kg following a 400 mg oral dose. Zonisamide, at concentrations of 1 to 7 mcg/mL, is approximately 40% bound to human plasma proteins. Zonisamide extensively binds to erythrocytes, resulting in an eight-fold higher concentration of zonisamide in red blood cells than in plasma. Protein binding of zonisamide is unaffected in the presence of therapeutic concentrations of phenytoin, phenobarbital or carbamazepine.
Metabolism and Elimination:
Following oral administration of 14C-zonisamide to healthy volunteers, only zonisamide was detected in plasma. Zonisamide is excreted primarily in urine as parent drug and as the glucuronide of a metabolite. Following multiple dosing, 62% of the radiolabeled dose was recovered in the urine, with 3% in the feces by day 10. Zonisamide undergoes acetylation by N-acetyl-transferases to form N-acetyl zonisamide and reduction to form the open ring metabolite, 2-sulfamoylacetyl phenol (SMAP). Of the excreted dose, 35% was recovered as zonisamide, 15% as N-acetyl zonisamide, and 50% as the glucuronide of SMAP. Reduction of zonisamide to SMAP is mediated by cytochrome P450 isozyme 3A4 (CYP3A4). Zonisamide does not induce its own metabolism. The plasma clearance of oral zonisamide is approximately 0.3 to 0.35 mL/min/kg in patients not receiving enzyme-inducing antiepilepsy drugs (AEDs). The clearance of zonisamide is increased to 0.5 mL/min/kg in patients concurrently on enzyme-inducing AEDs.
After a single-dose administration, renal clearance of zonisamide is approximately 3.5 mL/min. The clearance of an oral dose of zonisamide from red blood cells is 2 mL/min. The elimination half-life of zonisamide in plasma is approximately 63 hours. The elimination half-life of zonisamide in red blood cells is approximately 105 hours.
Specific Populations:
Renal Impairment: Single 300 mg zonisamide doses were administered to three groups of volunteers. Group 1 was a healthy group with a creatinine clearance ranging from 70 to 152 mL/min. Group 2 and Group 3 had creatinine clearances ranging from 14.5 to 59 mL/min and 10 to 20 mL/min, respectively. Zonisamide renal clearance decreased with decreasing renal function (3.42, 2.5, 2.23 mL/min, respectively). Marked renal impairment (creatinine clearance < 20 mL/min) was associated with an increase in zonisamide AUC of 35% (see DOSAGE AND ADMINISTRATION section).
Hepatic Impairment: The pharmacokinetics of zonisamide in patients with impaired liver function have not been studied (see DOSAGE AND ADMINISTRATION section).
Age: The pharmacokinetics of a 300 mg single dose of zonisamide was similar in young (mean age 28 years) and elderly subjects (mean age 69 years).
Gender and Race: Information on the effect of gender and race on the pharmacokinetics of zonisamide is not available.
Effects of zonisamide on cytochrome P450 enzymes
In vitro studies using human liver microsomes show insignificant (<25%) inhibition of cytochrome P450 isozymes 1A2, 2A6, 2C9, 2C19, 2D6, 2E1, 3A4, 2B6 or 2C8 at zonisamide levels approximately two-fold or greater than clinically relevant unbound serum concentrations. Therefore, zonisamide is not expected to affect the pharmacokinetics of other drugs via cytochrome P450-mediated mechanisms.
Potential for zonisamide to affect other drugs
Anti-epileptic drugs
In epileptic patients, steady-state dosing with zonisamide resulted in no clinically relevant pharmacokinetic effects on carbamazepine, lamotrigine, phenytoin, or sodium valproate.
Oral contraceptives
In healthy subjects, steady state dosing with zonisamide did not affect serum concentrations of ethinylestradiol or norethisterone in a combined oral contraceptive.
CYP2D6 substrates:
Coadministration of multiple dosing of zonisamide up to 400 mg/day with single 50 mg doses of desipramine did not significantly affect the pharmacokinetic parameters of desipramine, a probe drug for CYP2D6 activity.
P-gp substrate
An in vitro study showed that zonisamide is a weak inhibitor of P-gp (MDR1) with an IC50 of 267 μmol/L. There is a theoretical potential for zonisamide to affect the pharmacokinetics of drugs which are P-gp substrates.
Caution is advised when starting or stopping zonisamide or changing the zonisamide dose in patients who are also receiving drugs which are P-gp substrates (e.g., digoxin, quinidine)
Potential for Medicinal Products to Affect Zonisamide
Concomitant medications that can induce or inhibit CYP3A4 or N-acetyl-transferases may affect the pharmacokinetics of zonisamide. Drugs which inhibit or induce glucuronide conjugation are not expected to influence the pharmacokinetics of zonisamide.
The absence of a clinically significant pharmacokinetic interaction between zonisamide and lamotrigine indicates a low potential for zonisamide to interact with substances which are metabolized by UDP-GT.
CYP3A4 Induction: Drugs that induce liver enzymes increase the metabolism and clearance of zonisamide and decrease its half-life. The half-life of zonisamide following a 400 mg dose in patients concurrently on enzyme-inducing AEDs such as phenytoin, carbamazepine, or phenobarbital was between 27 to 38 hours, the half-life of zonisamide in patients concurrently on the non-enzyme inducing AED, valproate, was 46 hours.
These effects are unlikely to be of clinical significance when zonisamide is added to existing therapy; however, changes in zonisamide concentrations may occur if concomitant CYP3A4 inducing anti-epileptic or other drugs are withdrawn, dose adjusted or introduced, an adjustment of the zonisamide dose may be required. If coadministration with a potent CYP3A4 inducer (e.g., rifampicin) is necessary, the patient should be closely monitored and the dose of zonisamide and other drugs that are CYP3A4 substrate may need to be adjusted.
CYP3A4 Inhibition: Steady-state dosing of either ketoconazole (400 mg/day) or cimetidine (1,200 mg/day) had no clinically relevant effects on the single dose pharmacokinetics of zonisamide given to healthy subjects. Therefore, modification of zonisamide dosing is not necessary when coadministered with known CYP3A4 inhibitors.
Interactions of Zonisamide with Other Carbonic Anhydrase Inhibitors:
Concomitant use of zonisamide, a carbonic anhydrase inhibitor, with any other carbonic anhydrase inhibitor (e.g., topiramate, acetazolamide or dichlorphenamide), may increase the severity of metabolic acidosis and may also increase the risks of hyperammonemia and kidney stone formation. Therefore, if zonisamide is given concomitantly with another carbonic anhydrase inhibitor, the patient should be monitored for the appearance or worsening of metabolic acidosis (see WARNINGS, Metabolic Acidosis subsection and Hyperammonemia and Encephalopathy subsection and PRECAUTIONS, Drug Interactions subsection).
Clinical Studies: The effectiveness of zonisamide as adjunctive therapy (added to other antiepilepsy drugs) has been established in three multicenter, placebo-controlled, double blind, 3-month clinical trials (two domestic, one European) in 499 patients with refractory partial onset seizures with or without secondary generalization. Each patient had a history of at least four partial onset seizures per month in spite of receiving one or two antiepilepsy drugs at therapeutic concentrations. The 499 patients (209 women, 290 men) ranged in age from 13 to 68 years with a mean age of about 35 years. In the two U.S. studies, over 80% of patients were Caucasian; 100% of patients in the European study were Caucasian. Zonisamide or placebo was added to the existing therapy. The primary measure of effectiveness was median percent reduction from baseline in partial seizure frequency. The secondary measure was proportion of patients achieving a 50% or greater seizure reduction from baseline (responders). The results described below are for all partial seizures in the intent-to-treat populations.
In the first study (n = 203), all patients had a 1-month baseline observation period, then received placebo or zonisamide in one of two dose escalation regimens; either 1) 100 mg/day for five weeks, 200 mg/day for one week, 300 mg/day for one week, and then 400 mg/day for five weeks; or 2) 100 mg/day for one week, followed by 200 mg/day for five weeks, then 300 mg/day for one week, then 400 mg/day for five weeks. This design allowed a 100 mg vs. placebo comparison over weeks 1 to 5, and a 200 mg vs. placebo comparison over weeks 2 to 6; the primary comparison was 400 mg (both escalation groups combined) vs. placebo over weeks 8 to 12. The total daily dose was given as twice a day dosing. Statistically significant treatment differences favoring zonisamide were seen for doses of 100, 200, and 400 mg/day.
In the second (n = 152) and third (n = 138) studies, patients had a 2 to 3 month baseline, then were randomly assigned to placebo or zonisamide for three months. Zonisamide was introduced by administering 100 mg/day for the first week, 200 mg/day the second week, then 400 mg/day for two weeks, after which the dose (zonisamide or placebo) could be adjusted as necessary to a maximum dose of 20 mg/kg/day or a maximum plasma level of 40 mcg/mL. In the second study, the total daily dose was given as twice a day dosing; in the third study, it was given as a single daily dose. The average final maintenance doses received in the studies were 530 and 430 mg/day in the second and third studies, respectively. Both studies demonstrated statistically significant differences favoring zonisamide for doses of 400 to 600 mg/day, and there was no apparent difference between once daily and twice daily dosing (in different studies). Analysis of the data (first 4 weeks) during titration demonstrated statistically significant differences favoring zonisamide at doses between 100 and 400 mg/day. The primary comparison in both trials was for any dose over Weeks 5 to 12.
Table 1. Median % Reduction in All Partial Seizures and % Responders in Primary Efficacy Analyses: Intent-To-Treat Analysis - *
- p<0.05 compared to placebo
Study
Median % reduction in partial seizures
% Responders
Zonisamide
Placebo
Zonisamide
Placebo
Study 1:
Weeks 8 to 12:
n=98
40.5%*
n=72
9%
n=98
41.8%*
n=72
22.2%
Study 2:
Weeks 5 to 12:
n=69
29.6%*
n=72
-3.2%
n=69
29%
n=72
15%
Study 3:
Weeks 5 to 12:
n=67
27.2%*
n=66
-1.1%
n=67
28%*
n=66
12%
Table 2. Median % Reduction in All Partial Seizures and % Responders for Dose Analyses in Study 1: Intent-To-Treat Analysis - *
- p<0.05 compared to placebo
Dose Group
Median % reduction in partial seizures
% Responders
Zonisamide
Placebo
Zonisamide
Placebo
100 to 400 mg/day:
Weeks 1 to 12:
n=112
32.3%*
n=83
5.6%
n=112
32.1%*
n=83
9.6%
100 mg/day:
Weeks 1 to 5:
n=56
24.7%*
n=80
8.3%
n=56
25%*
n=80
11.3%
200 mg/day:
Weeks 2 to 6:
n=55
20.4%*
n=82
4%
n=55
25.5%*
n=82
9.8%
Figure 1 presents the proportion of patients (X-axis) whose percentage reduction from baseline in the all partial seizure rate was at least as great as that indicated on the Y-axis in the second and third placebo-controlled trials. A positive value on the Y-axis indicates an improvement from baseline (i.e., a decrease in seizure rate), while a negative value indicates a worsening from baseline (i.e., an increase in seizure rate). Thus, in a display of this type, the curve for an effective treatment is shifted to the left of the curve for placebo. The proportion of patients achieving any particular level of reduction in seizure rate was consistently higher for the zonisamide groups compared to the placebo groups. For example, Figure 1 indicates that approximately 27% of patients treated with zonisamide experienced a 75% or greater reduction, compared to approximately 12% in the placebo groups.
Close
Figure 1 Proportion of Patients Achieving Differing Levels of Seizure Reduction in Zonisamide and Placebo Groups in Studies 2 and 3
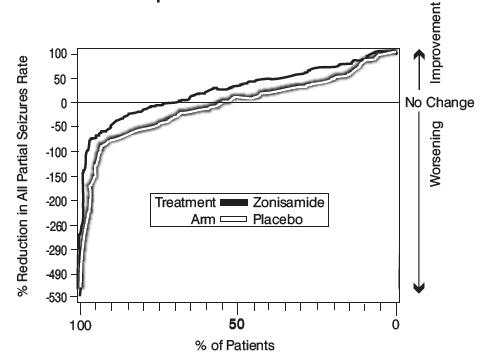
No differences in efficacy based on age, sex or race, as measured by a change in seizure frequency from baseline, were detected. -
INDICATIONS AND USAGEZonisamide is indicated as adjunctive therapy in the treatment of partial seizures in adults with epilepsy.
Zonisamide is indicated as adjunctive therapy in the treatment of partial seizures in adults with epilepsy.
Close -
CONTRAINDICATIONSZonisamide is contraindicated in patients who have demonstrated hypersensitivity to sulfonamides or zonisamide.
Zonisamide is contraindicated in patients who have demonstrated hypersensitivity to sulfonamides or zonisamide.
Close -
WARNINGSPotentially Fatal Reactions to Sulfonamides: Fatalities have occurred, although rarely, as a result of severe reactions to sulfonamides (zonisamide is a sulfonamide) including Stevens-Johnson ...
Potentially Fatal Reactions to Sulfonamides: Fatalities have occurred, although rarely, as a result of severe reactions to sulfonamides (zonisamide is a sulfonamide) including Stevens-Johnson syndrome, toxic epidermal necrolysis, fulminant hepatic necrosis, agranulocytosis, aplastic anemia, and other blood dyscrasias. Such reactions may occur when a sulfonamide is readministered irrespective of the route of administration. If signs of hypersensitivity or other serious reactions occur, discontinue zonisamide immediately. Specific experience with sulfonamide-type adverse reaction to zonisamide is described below.
Serious Skin Reactions:
Consideration should be given to discontinuing zonisamide in patients who develop an otherwise unexplained rash. If the drug is not discontinued, patients should be observed frequently. Seven deaths from severe rash [i.e., Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN)] were reported in the first 11 years of marketing in Japan. All of the patients were receiving other drugs in addition to zonisamide. In postmarketing experience from Japan, a total of 49 cases of SJS or TEN have been reported, a reporting rate of 46 per million patient-years of exposure. Although this rate is greater than background, it is probably an underestimate of the true incidence because of under-reporting. There were no confirmed cases of SJS or TEN in the U.S., European, or Japanese development programs.
In the U.S. and European randomized controlled trials, 6 of 269 (2.2%) zonisamide patients discontinued treatment because of rash compared to none on placebo. Across all trials during the U.S. and European development, rash that led to discontinuation of zonisamide was reported in 1.4% of patients (12 events per 1,000 patient-years of exposure). During Japanese development, serious rash or rash that led to study drug discontinuation was reported in 2% of patients (27.8 events per 1,000 patient-years). Rash usually occurred early in treatment, with 85% reported within 16 weeks in the U.S. and European studies and 90% reported within two weeks in the Japanese studies. There was no apparent relationship of dose to the occurrence of rash.
Serious Hematologic Events:
Two confirmed cases of aplastic anemia and one confirmed case of agranulocytosis were reported in the first 11 years of marketing in Japan, rates greater than generally accepted background rates. There were no cases of aplastic anemia and two confirmed cases of agranulocytosis in the U.S., European, or Japanese development programs. There is inadequate information to assess the relationship, if any, between dose and duration of treatment and these events.
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multi-Organ Hypersensitivity:
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS), also known as multi-organ hypersensitivity, has occurred with zonisamide. Some of these events have been fatal or life-threatening. DRESS typically, although not exclusively, presents with fever, rash, lymphadenopathy and/or facial swelling, in association with other organ system involvement, such as hepatitis, nephritis, hematologic abnormalities, myocarditis, or myositis, sometimes resembling an acute viral infection. Eosinophilia is often present. This disorder is variable in its expression, and other organ systems not noted here may be involved. It is important to note that early manifestations of hypersensitivity (e.g., fever, lymphadenopathy) may be present even though rash is not evident. If such signs or symptoms are present, the patient should be evaluated immediately. Zonisamide should be discontinued if an alternative etiology for the signs or symptoms cannot be established.
Oligohidrosis and Hyperthermia in Pediatric Patients:
Oligohidrosis, sometimes resulting in heat stroke and hospitalization, is seen in association with zonisamide in pediatric patients.
During the pre-approval development program in Japan, one case of oligohidrosis was reported in 403 pediatric patients, an incidence of 1 case per 285 patient-years of exposure. While there were no cases reported in the U.S. or European development programs, fewer than 100 pediatric patients participated in these trials.
In the first 11 years of marketing in Japan, 38 cases were reported, an estimated reporting rate of about 1 case per 10,000 patient-years of exposure. In the first year of marketing in the U.S., 2 cases were reported, an estimated reporting rate of about 12 cases per 10,000 patient-years of exposure. These rates are underestimates of the true incidence because of under-reporting. There has also been one report of heat stroke in an 18-year-old patient in the U.S.
Decreased sweating and an elevation in body temperature above normal characterized these cases. Many cases were reported after exposure to elevated environmental temperatures. Heat stroke, requiring hospitalization, was diagnosed in some cases. There have been no reported deaths.
Pediatric patients appear to be at an increased risk for zonisamide-associated oligohidrosis and hyperthermia. Patients, especially pediatric patients, treated with zonisamide should be monitored closely for evidence of decreased sweating and increased body temperature, especially in warm or hot weather. Caution should be used when zonisamide is prescribed with other drugs that predispose patients to heat-related disorders; these drugs include, but are not limited to, carbonic anhydrase inhibitors and drugs with anticholinergic activity.
The practitioner should be aware that the safety and effectiveness of zonisamide in pediatric patients have not been established, and that zonisamide is not approved for use in pediatric patients.
Acute Myopia and Secondary Angle Closure Glaucoma:
Acute myopia and secondary angle closure glaucoma have been reported in patients receiving zonisamide. Elevated intraocular pressure can lead to serious sequelae, including permanent vision loss, if left untreated.
Symptoms in reported cases have included acute onset of decreased visual acuity and/or ocular pain. Ophthalmologic findings can include myopia, anterior chamber shallowing, ocular hyperemia (redness), and increased intraocular pressure. Mydriasis may or may not be present. This syndrome may be associated with ciliochoroidal effusion resulting in anterior displacement of the lens and iris, with secondary angle closure glaucoma. Symptoms typically occur within one month after initiating zonisamide therapy.
In contrast to primary narrow angle glaucoma, which is rare under 40 years of age, secondary angle closure glaucoma associated with zonisamide has been reported both in pediatric patients and in adults. The primary treatment to reverse symptoms is discontinuation of zonisamide as rapidly as possible, according to the judgment of the treating physician. Other therapeutic measures, in conjunction with discontinuation of zonisamide, may be helpful. Myopia and secondary angle closure glaucoma usually resolve or improve after discontinuation of zonisamide.
Suicidal Behavior and Ideation:
Antiepileptic drugs (AEDs), including zonisamide, increase the risk of suicidal thoughts or behavior in patients taking these drugs for any indication. Patients treated with any AED for any indication should be monitored for the emergence or worsening of depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior.
Pooled analyses of 199 placebo-controlled clinical trials (mono- and adjunctive therapy) of 11 different AEDs showed that patients randomized to one of the AEDs had approximately twice the risk (adjusted Relative Risk 1.8, 95% CI:1.2, 2.7) of suicidal thinking or behavior compared to patients randomized to placebo. In these trials, which had a median treatment duration of 12 weeks, the estimated incidence rate of suicidal behavior or ideation among 27,863 AED-treated patients was 0.43%, compared to 0.24% among 16,029 placebo-treated patients, representing an increase of approximately one case of suicidal thinking or behavior for every 530 patients treated. There were four suicides in drug-treated patients in the trials and none in placebo-treated patients, but the number is too small to allow any conclusion about drug effect on suicide.
The increased risk of suicidal thoughts or behavior with AEDs was observed as early as one week after starting drug treatment with AEDs and persisted for the duration of treatment assessed. Because most trials included in the analysis did not extend beyond 24 weeks, the risk of suicidal thoughts or behavior beyond 24 weeks could not be assessed.
The risk of suicidal thoughts or behavior was generally consistent among drugs in the data analyzed. The finding of increased risk with AEDs of varying mechanisms of action and across a range of indications suggests that the risk applies to all AEDs used for any indication. The risk did not vary substantially by age (5 to 100 years) in the clinical trials analyzed.
Table 3 shows absolute and relative risk by indication for all evaluated AEDs.
Table 3. Risk by indication for antiepileptic drugs in the pooled analysis Indication
Placebo
Patients with Events Per 1,000 Patients
Drug Patients with Events Per 1,000 Patients
Relative Risk: Incidence of Events in Drug
Patients/Incidence in Placebo Patients
Risk Difference: Additional Drug Patients with Events Per
1,000 Patients
Epilepsy
1
3.4
3.5
2.4
Psychiatric
5.7
8.5
1.5
2.9
Other
1
1.8
1.9
0.9
Total
2.4
4.3
1.8
1.9
The relative risk for suicidal thoughts or behavior was higher in clinical trials for epilepsy than in clinical trials for psychiatric or other conditions, but the absolute risk differences were similar for the epilepsy and psychiatric indications.
Anyone considering prescribing zonisamide or any other AED must balance the risk of suicidal thoughts or behavior with the risk of untreated illness. Epilepsy and many other illnesses for which AEDs are prescribed are themselves associated with morbidity and mortality and an increased risk of suicidal thoughts and behavior. Should suicidal thoughts and behavior emerge during treatment, the prescriber needs to consider whether the emergence of these symptoms in any given patient may be related to the illness being treated.
Patients, their caregivers, and families should be informed that AEDs increase the risk of suicidal thoughts and behavior and should be advised of the need to be alert for the emergence or worsening of the signs and symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts about self-harm. Behaviors of concern should be reported immediately to healthcare providers (see WARNINGS, Cognitive/Neuropsychiatric Adverse Events subsection below).
Metabolic Acidosis: Zonisamide causes hyperchloremic, non-anion gap, metabolic acidosis (i.e., decreased serum bicarbonate below the normal reference range in the absence of chronic respiratory alkalosis) (see PRECAUTIONS, Laboratory Tests subsection). This metabolic acidosis is caused by renal bicarbonate loss due to the inhibitory effect of zonisamide on carbonic anhydrase.
Generally, zonisamide-induced metabolic acidosis occurs early in treatment, but it can develop at any time during treatment. Metabolic acidosis generally appears to be dose-dependent and can occur at doses as low as 25 mg daily.
Conditions or therapies that predispose to acidosis (such as renal disease, severe respiratory disorders, status epilepticus, diarrhea, ketogenic diet, or specific drugs) may be additive to the bicarbonate lowering effects of zonisamide.
Some manifestations of acute or chronic metabolic acidosis include hyperventilation, nonspecific symptoms such as fatigue and anorexia, or more severe sequelae including cardiac arrhythmias or stupor. Chronic, untreated, metabolic acidosis may increase the risk for nephrolithiasis or nephrocalcinosis. Nephrolithiasis has been observed in the clinical development program in 4 % of adults treated with zonisamide, has also been detected by renal ultrasound in 8 % of pediatric treated patients who had at least one ultrasound prospectively collected, and was reported as an adverse event in 3 % (4/133) of pediatric patients (see PRECAUTIONS, Kidney Stones subsection). Metabolic acidosis can also increase the risk for hyperammonemia, particularly in the presence of drugs which can cause hyperammonemia.
Chronic, untreated metabolic acidosis may result in osteomalacia (referred to as rickets in pediatric patients) and/or osteoporosis with an increased risk for fracture. Of potential relevance, zonisamide treatment was associated with reductions in serum phosphorus and increases in serum alkaline phosphatase, changes that may be related to metabolic acidosis and osteomalacia (see PRECAUTIONS, Laboratory Tests subsection).
Chronic, untreated metabolic acidosis in pediatric patients may reduce growth rates. A reduction in growth rate may eventually decrease the maximal height achieved. The effect of zonisamide on growth and bone-related sequelae has not been systematically investigated.
Measurement of baseline and periodic serum bicarbonate during treatment is recommended. If metabolic acidosis develops and persists, consideration should be given to reducing the dose or discontinuing zonisamide (using dose tapering). If the decision is made to continue patients on zonisamide in the face of persistent acidosis, alkali treatment should be considered.
Serum bicarbonate was not measured in the adjunctive controlled trials of adults with epilepsy. However, serum bicarbonate was studied in three clinical trials for indications which have not been approved: a placebo-controlled trial for migraine prophylaxis in adults, a controlled trial for monotherapy in epilepsy in adults, and an open label trial for adjunctive treatment of epilepsy in pediatric patients (3 to 16 years). In adults, mean serum bicarbonate reductions ranged from approximately 2 mEq/L at daily doses of 100 mg to nearly 4 mEq/L at daily doses of 300 mg. In pediatric patients, mean serum bicarbonate reductions ranged from approximately 2 mEq/L at daily doses from above 100 mg up to 300 mg, to nearly 4 mEq/L at daily doses from above 400 mg up to 600 mg.
In two controlled studies in adults, the incidence of a persistent treatment-emergent decrease in serum bicarbonate to less than 20 mEq/L (observed at 2 or more consecutive visits or the final visit) was dose-related at relatively low zonisamide doses. In the monotherapy trial of epilepsy, the incidence of a persistent treatment-emergent decrease in serum bicarbonate was 21% for daily zonisamide doses of 25 mg or 100 mg, and was 43% at a daily dose of 300 mg. In a placebo-controlled trial for prophylaxis of migraine, the incidence of a persistent treatment-emergent decrease in serum bicarbonate was 7% for placebo, 29% for 150 mg daily, and 34% for 300 mg daily. The incidence of persistent markedly abnormally low serum bicarbonate (decrease to less than 17 mEq/L and more than 5 mEq/L from a pretreatment value of at least 20 mEq/L) in these controlled trials was 2% or less.
In the pediatric study, the incidence of persistent, treatment-emergent decreases in serum bicarbonate to levels less than 20 mEq/L was 52% at doses up to 100 mg daily, was 90% for a wide range of doses up to 600 mg daily, and generally appeared to increase with higher doses. The incidence of a persistent markedly abnormally low serum bicarbonate value was 4 % at doses up to 100 mg daily, was 18% for a wide range of doses up to 600 mg daily, and generally appeared to increase with higher doses. Some patients experienced moderately severe serum bicarbonate decrements down to a level as low as 10 mEq/L. The relatively high frequencies of varying severities of metabolic acidosis observed in this study of pediatric patients (compared to the frequency and severity observed in various clinical trial development programs in adults) suggest that pediatric patients may be more likely to develop metabolic acidosis than adults.
Seizures on Withdrawal:
As with other AEDs, abrupt withdrawal of zonisamide in patients with epilepsy may precipitate increased seizure frequency or status epilepticus. Dose reduction or discontinuation of zonisamide should be done gradually.
Teratogenicity:
Women of child bearing potential who are given zonisamide should be advised to use effective contraception. Zonisamide was teratogenic in mice, rats, and dogs and embryolethal in monkeys when administered during the period of organogenesis. A variety of fetal abnormalities, including cardiovascular defects, and embryo-fetal deaths occurred at maternal plasma levels similar to or lower than therapeutic levels in humans. These findings suggest that the use of zonisamide during pregnancy in humans may present a significant risk to the fetus (see PRECAUTIONS, Pregnancy subsection). Zonisamide should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Cognitive/Neuropsychiatric Adverse Events:
Use of zonisamide was frequently associated with central nervous system-related adverse events. The most significant of these can be classified into three general categories: 1) psychiatric symptoms, including depression and psychosis, 2) psychomotor slowing, difficulty with concentration, and speech or language problems, in particular, word-finding difficulties, and 3) somnolence or fatigue.
In placebo-controlled trials, 2.2% of patients discontinued zonisamide or were hospitalized for depression compared to 0.4% of placebo patients. Among all epilepsy patients treated with zonisamide, 1.4% were discontinued and 1% were hospitalized because of reported depression or suicide attempts. In placebo-controlled trials, 2.2% of patients discontinued zonisamide or were hospitalized due to psychosis or psychosis-related symptoms compared to none of the placebo patients. Among all epilepsy patients treated with zonisamide, 0.9% were discontinued and 1.4% were hospitalized because of reported psychosis or related symptoms.
Psychomotor slowing and difficulty with concentration occurred in the first month of treatment and were associated with doses above 300 mg/day. Speech and language problems tended to occur after 6 to 10 weeks of treatment and at doses above 300 mg/day. Although in most cases these events were of mild to moderate severity, they at times led to withdrawal from treatment.
Somnolence and fatigue were frequently reported CNS adverse events during clinical trials with zonisamide. Although in most cases these events were of mild to moderate severity, they led to withdrawal from treatment in 0.2% of the patients enrolled in controlled trials. Somnolence and fatigue tended to occur within the first month of treatment. Somnolence and fatigue occurred most frequently at doses of 300 to 500 mg/day. Patients should be cautioned about this possibility and special care should be taken by patients if they drive, operate machinery, or perform any hazardous task.
Hyperammonemia and Encephalopathy:
Hyperammonemia and encephalopathy have been reported with the postmarketing use of zonisamide. Zonisamide treatment inhibits carbonic anhydrase activity, which may cause metabolic acidosis that is associated with an increased risk for developing hyperammonemia. Hyperammonemia resulting from zonisamide can also be asymptomatic.
The risks of hyperammonemia and various manifestations of encephalopathy may be increased in patients treated with zonisamide and concomitantly taking other medications that can cause hyperammonemia, including valproic acid or topiramate (see PRECAUTIONS). Patients with inborn errors of metabolism or reduced hepatic mitochondrial activity may be at an increased risk for hyperammonemia with or without encephalopathy and this risk may be increased by zonisamide use.
Measure serum ammonia concentration if signs or symptoms (e.g., unexplained change in mental status, vomiting, or lethargy) of encephalopathy occur. Hyperammonemia resulting from zonisamide resolves when zonisamide is discontinued. Hyperammonemia from zonisamide may resolve or decrease in severity with a decrease of the daily dose.
Close -
PRECAUTIONSGeneral: Somnolence is commonly reported, especially at higher doses of zonisamide (see WARNINGS: Cognitive/Neuropsychiatric Adverse Events subsection). Zonisamide is metabolized by the liver ...
General:
Somnolence is commonly reported, especially at higher doses of zonisamide (see WARNINGS: Cognitive/Neuropsychiatric Adverse Events subsection). Zonisamide is metabolized by the liver and eliminated by the kidneys; caution should therefore be exercised when administering zonisamide to patients with hepatic and renal dysfunction (see CLINICAL PHARMACOLOGY, Specific Populations subsection).
Kidney Stones:
Among 991 patients treated during the development of zonisamide, 40 patients (4%) with epilepsy receiving zonisamide developed clinically possible or confirmed kidney stones (e.g., clinical symptomatology, sonography, etc.), a rate of 34 per 1,000 patient-years of exposure (40 patients with 1,168 years of exposure). Of these, 12 were symptomatic, and 28 were described as possible kidney stones based on sonographic detection. In nine patients, the diagnosis was confirmed by a passage of a stone or by a definitive sonographic finding. The rate of occurrence of kidney stones was 28.7 per 1,000 patient-years of exposure in the first six months, 62.6 per 1,000 patient-years of exposure between 6 and 12 months, and 24.3 per 1,000 patient-years of exposure after 12 months of use. There are no normative sonographic data available for either the general population or patients with epilepsy. Although the clinical significance of the sonographic findings may not be certain, the development of nephrolithiasis may be related to metabolic acidosis (see WARNINGS, Metabolic Acidosis subsection). The analyzed stones were composed of calcium or urate salts. In general, increasing fluid intake and urine output can help reduce the risk of stone formation, particularly in those with predisposing risk factors. It is unknown, however, whether these measures will reduce the risk of stone formation in patients treated with zonisamide.
Although not approved in pediatric patients, sonographic findings consistent with nephrolithiasis were also detected in 8 % of a subset of zonisamide treated pediatric patients who had at least one renal ultrasound prospectively performed in a clinical development program investigating open-label treatment. The incidence of kidney stone as an adverse event was 3 % (see WARNINGS, Metabolic Acidosis subsection).
Effect on Renal Function: In several clinical studies, zonisamide was associated with a statistically significant 8% mean increase from baseline of serum creatinine and blood urea nitrogen (BUN) compared to essentially no change in the placebo patients. The increase appeared to persist over time but was not progressive; this has been interpreted as an effect on glomerular filtration rate (GFR). There were no episodes of unexplained acute renal failure in clinical development in the U.S., Europe, or Japan. The decrease in GFR appeared within the first 4 weeks of treatment. In a 30-day study, the GFR returned to baseline within 2 to 3 weeks of drug discontinuation. There is no information about reversibility, after drug discontinuation, of the effects on GFR after long-term use. Zonisamide should be discontinued in patients who develop acute renal failure or a clinically significant sustained increase in the creatinine/BUN concentration. Zonisamide should not be used in patients with renal failure (estimated GFR < 50 mL/min) as there has been insufficient experience concerning drug dosing and toxicity.
Status Epilepticus: Estimates of the incidence of treatment emergent status epilepticus in zonisamide-treated patients are difficult because a standard definition was not employed. Nonetheless, in controlled trials, 1.1% of patients treated with zonisamide had an event labeled as status epilepticus compared to none of the patients treated with placebo. Among patients treated with zonisamide across all epilepsy studies (controlled and uncontrolled), 1% of patients had an event reported as status epilepticus.
Information for Patients:
Inform patients of the availability of a Medication Guide, and instruct them to read the Medication Guide prior to taking zonisamide capsules. Instruct patients to take zonisamide capsules only as prescribed.
Advise patients as follows: (See Medication Guide)
- Zonisamide may produce drowsiness, especially at higher doses. Patients should be advised not to drive a car or operate other complex machinery until they have gained experience on zonisamide sufficient to determine whether it affects their performance. Because of the potential of zonisamide to cause CNS depression, as well as other cognitive and/or neuropsychiatric adverse events, zonisamide should be used with caution if used in combination with alcohol or other CNS depressants.
- Patients should contact their physician immediately if a skin rash develops (see WARNINGS, Serious Skin Reactions subsection).
- Instruct patients to seek immediate medical attention if they experience blurred vision, visual disturbances, or periorbital pain (see WARNINGS, Acute Myopia and Secondary Angle Closure Glaucoma subsection).
- Patients should contact their physician immediately if they develop signs or symptoms, such as sudden back pain, abdominal pain, and/or blood in the urine, that could indicate a kidney stone. Increasing fluid intake and urine output may reduce the risk of stone formation, particularly in those with predisposing risk factors for stones (see PRECAUTIONS, Kidney Stones subsection).
- Patients should contact their physician immediately if a child has been taking zonisamide and is not sweating as usual with or without a fever (see WARNINGS, Oligohidrosis and Hyperthermia in Pediatric Patients subsection).
- Because zonisamide can cause hematological complications, patients should contact their physician immediately if they develop a fever, sore throat, oral ulcers, or easy bruising (see WARNINGS, Serious Hematologic Events subsection).
- Counsel patients and caregivers that AEDs, including zonisamide, may increase the risk of suicidal thoughts and behavior and advise them of the need to be alert for the emergence or worsening of symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts about self-harm. Behaviors of concern should be reported immediately to healthcare providers (see WARNINGS, Suicidal Behavior and Ideation subsection).
- Warn patients about the possible development of hyperammonemia with or without encephalopathy. Although hyperammonemia may be asymptomatic, clinical symptoms of hyperammonemic encephalopathy often include acute alterations in level of consciousness and/or cognitive function with lethargy and/or vomiting. Instruct patients to contact their physician if they develop unexplained lethargy, vomiting, or changes in mental status (see WARNINGS, Hyperammonemia and Encephalopathy subsection)
- Patients should contact their physician immediately if they develop fast breathing, fatigue/tiredness, loss of appetite, or irregular heart beat or palpitations, which are possible manifestations of metabolic acidosis (see WARNINGS, Metabolic Acidosis subsection).
- As with other AEDs, patients should contact their physician if they intend to become pregnant or are pregnant during zonisamide therapy. Patients should notify their physician if they intend to breast-feed or are breast-feeding an infant (see PRECAUTIONS, Use in Nursing Mothers subsection).
- Encourage patients to enroll in the North American Antiepileptic Drug (NAAED) Pregnancy Registry if they become pregnant. This registry is collecting information about the safety of antiepileptic drugs during pregnancy. To enroll, patients can call the toll-free number 1-888-233-2334 (see PRECAUTIONS, Pregnancy subsection).
Laboratory Tests:
In several clinical studies, zonisamide was associated with a mean increase in the concentration of serum creatinine and blood urea nitrogen (BUN) of approximately 8% over the baseline measurement. Consideration should be given to monitoring renal function periodically (see PRECAUTIONS, Effect on Renal Function subsection).
Zonisamide increases serum chloride and alkaline phosphatase and decreases serum bicarbonate (see WARNINGS, Metabolic Acidosis subsection), phosphorus, calcium, and albumin.
Drug Interactions with CNS Depressants: Concomitant administration of zonisamide and alcohol or other CNS depressant drugs has not been evaluated in clinical studies. Because of the potential of zonisamide to cause CNS depression, as well as other cognitive and/or neuropsychiatric adverse events, zonisamide should be used with caution if used in combination with alcohol or other CNS depressants.
Other Carbonic Anhydrase Inhibitors: Concomitant use of zonisamide, a carbonic anhydrase inhibitor, with any other carbonic anhydrase inhibitor (e.g., topiramate, acetazolamide or dichlorphenamide), may increase the severity of metabolic acidosis and may also increase the risk of kidney stone formation or the risk of hyperammonemia. Therefore, if zonisamide is given concomitantly with another carbonic anhydrase inhibitor, the patient should be monitored for the appearance or worsening of metabolic acidosis (see CLINICAL PHARMACOLOGY, Interactions of Zonisamide with Other Carbonic Anhydrase Inhibitors subsection and WARNINGS, Metabolic Acidosis subsection and Hyperammonemia and Encephalopathy subsection).
Carcinogenicity, Mutagenesis, Impairment of Fertility:
No evidence of carcinogenicity was found in mice or rats following dietary administration of zonisamide for two years at doses of up to 80 mg/kg/day. In mice, this dose is approximately equivalent to the maximum recommended human dose (MRHD) of 400 mg/day on a mg/m2 basis. In rats, this dose is 1 to 2 times the MRHD on a mg/m2 basis.
Zonisamide was mutagenic in an in vitro chromosomal aberration assay in CHL cells. Zonisamide was not mutagenic or clastogenic in other in vitro assays (Ames, mouse lymphoma tk assay, chromosomal aberration in human lymphocytes) or in the in vivo rat bone marrow cytogenetics assay.
Rats treated with zonisamide (20, 60, or 200 mg/kg) before mating and during the initial gestation phase showed signs of reproductive toxicity (decreased corpora lutea, implantations, and live fetuses) at all doses. The low dose in this study is approximately 0.5 times the maximum recommended human dose (MRHD) on a mg/m2 basis.
Pregnancy :
(see WARNINGS, Teratogenicity subsection):
Zonisamide may cause serious adverse fetal effects, based on clinical and nonclinical data. Zonisamide was teratogenic in multiple animal species.
Zonisamide treatment causes metabolic acidosis in humans. The effect of zonisamide-induced metabolic acidosis has not been studied in pregnancy; however, metabolic acidosis in pregnancy (due to other causes) may be associated with decreased fetal growth, decreased fetal oxygenation, and fetal death, and may affect the fetus's ability to tolerate labor. Pregnant patients should be monitored for metabolic acidosis and treated as in the non-pregnant state. (See WARNINGS, Metabolic Acidosis subsection.)
Newborns of mothers treated with zonisamide should be monitored for metabolic acidosis because of transfer of zonisamide to the fetus and possible occurrence of transient metabolic acidosis following birth. Transient metabolic acidosis has been reported in neonates born to mothers treated during pregnancy with a different carbonic anhydrase inhibitor.
Zonisamide was teratogenic in mice, rats, and dogs and embryolethal in monkeys when administered during the period of organogenesis. Fetal abnormalities or embryo-fetal deaths occurred in these species at zonisamide dosage and maternal plasma levels similar to or lower than therapeutic levels in humans, indicating that use of this drug in pregnancy entails a significant risk to the fetus. A variety of external, visceral, and skeletal malformations was produced in animals by prenatal exposure to zonisamide. Cardiovascular defects were prominent in both rats and dogs.
Following administration of zonisamide (10, 30, or 60 mg/kg/day) to pregnant dogs during organogenesis, increased incidences of fetal cardiovascular malformations (ventricular septal defects, cardiomegaly, various valvular and arterial anomalies) were found at doses of 30 mg/kg/day or greater. The low effect dose for malformations produced peak maternal plasma zonisamide levels (25 mcg/mL) about 0.5 times the highest plasma levels measured in patients receiving the maximum recommended human dose (MRHD) of 400 mg/day. In dogs, cardiovascular malformations were found in approximately 50% of all fetuses exposed to the high dose, which was associated with maternal plasma levels (44 mcg/mL) approximately equal to the highest levels measured in humans receiving the MRHD. Incidences of skeletal malformations were also increased at the high dose, and fetal growth retardation and increased frequencies of skeletal variations were seen at all doses in this study. The low dose produced maternal plasma levels (12 mcg/mL) about 0.25 times the highest human levels.
In cynomolgus monkeys, administration of zonisamide (10 or 20 mg/kg/day) to pregnant animals during organogenesis resulted in embryo-fetal deaths at both doses. The possibility that these deaths were due to malformations cannot be ruled out. The lowest embryolethal dose in monkeys was associated with peak maternal plasma zonisamide levels (5 mcg/mL) approximately 0.1 times the highest levels measured in patients at the MRHD.
In a mouse embryo-fetal development study, treatment of pregnant animals with zonisamide (125, 250, or 500 mg/kg/day) during the period of organogenesis resulted in increased incidences of fetal malformations (skeletal and/or craniofacial defects) at all doses tested. The low dose in this study is approximately 1.5 times the MRHD on a mg/m2 basis. In rats, increased frequencies of malformations (cardiovascular defects) and variations (persistent cords of thymic tissue, decreased skeletal ossification) were observed among the offspring of dams treated with zonisamide (20, 60, or 200 mg/kg/day) throughout organogenesis at all doses. The low effect dose is approximately 0.5 times the MRHD on a mg/m2 basis.
Perinatal death was increased among the offspring of rats treated with zonisamide (10, 30, or 60 mg/kg/day) from the latter part of gestation up to weaning at the high dose, or approximately 1.4 times the MRHD on a mg/m2 basis. The no effect level of 30 mg/kg/day is approximately 0.7 times the MRHD on a mg/m2 basis.
There are no adequate and well-controlled studies in pregnant women. Zonisamide should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
To provide information regarding the effects of in utero exposure to zonisamide, physicians are advised to recommend that pregnant patients taking zonisamide capsules enroll in the NAAED Pregnancy Registry. This can be done by calling the toll free number 1-888-233-2334, and must be done by patients themselves. Information on the registry can also be found at the website http://www.aedpregnancyregistry.org/.
Use in Nursing Mothers:
Zonisamide is excreted in human milk. Because of the potential for serious adverse reactions in nursing infants from zonisamide, a decision should be made whether to discontinue nursing or to discontinue drug, taking into account the importance of the drug to the mother.
Pediatric Use:
The safety and effectiveness of zonisamide in children under age 16 have not been established. Acute myopia and secondary angle closure glaucoma have been reported in pediatric patients (see WARNINGS, Acute Myopia and Secondary Angle Closure Glaucoma subsection). Cases of oligohidrosis and hyperpyrexia have been reported (see WARNINGS, Oligohidrosis and Hyperthermia in Pediatric Patients subsection). Zonisamide commonly causes metabolic acidosis in pediatric patients (see WARNINGS, Metabolic Acidosis subsection). Hyperammonemia with encephalopathy has been reported in pediatric patients (see WARNINGS, Hyperammonemia and Encephalopathy subsection). Chronic untreated metabolic acidosis in pediatric patients may cause nephrolithiasis and/or nephrocalcinosis, osteoporosis and/or osteomalacia (potentially resulting in rickets), and may reduce growth rates. A reduction in growth rate may eventually decrease the maximal height achieved. The effect of zonisamide on growth and bone-related sequelae has not been systematically investigated.
CloseGeriatric Use:
Single dose pharmacokinetic parameters are similar in elderly and young healthy volunteers (see CLINICAL PHARMACOLOGY, Specific Populations subsection). Clinical studies of zonisamide did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
-
ADVERSE REACTIONSThe most common adverse reactions with zonisamide (an incidence at least 4% greater than placebo) in controlled clinical trials and shown in descending order of frequency were somnolence ...
The most common adverse reactions with zonisamide (an incidence at least 4% greater than placebo) in controlled clinical trials and shown in descending order of frequency were somnolence, anorexia, dizziness, ataxia, agitation/irritability, and difficulty with memory and/or concentration.
In controlled clinical trials, 12% of patients receiving zonisamide as adjunctive therapy discontinued due to an adverse reaction compared to 6% receiving placebo. Approximately 21% of the 1,336 patients with epilepsy who received zonisamide in clinical studies discontinued treatment because of an adverse reaction. The most common adverse reactions leading to discontinuation were somnolence, fatigue and/or ataxia (6%), anorexia (3%), difficulty concentrating (2%), difficulty with memory, mental slowing, nausea/vomiting (2%), and weight loss (1%). Many of these adverse reactions were dose-related (see WARNINGS and PRECAUTIONS).
Adverse Reaction Incidence in Controlled Clinical Trials:
Table 4 lists adverse reactions that occurred in at least 2% of patients treated with zonisamide in controlled clinical trials that were numerically more common in the zonisamide group. In these studies, either zonisamide or placebo was added to the patient's current AED therapy.
Table 4. Adverse Reactions in Placebo-Controlled, Add-On Trials (Events that occurred in at least 2% of Zonisamide-treated patients and occurred more frequently in Zonisamide -treated than placebo-treated patients) BODY SYSTEM/PREFERRED TERM
ZONISAMIDE
(n=269) %
PLACEBO (n=230) %
BODY AS A WHOLE
Headache
10
8
Abdominal Pain
6
3
Flu Syndrome
4
3
DIGESTIVE
Anorexia
13
6
Nausea
9
6
Diarrhea
5
2
Dyspepsia
3
1
Constipation
2
1
Dry Mouth
2
1
HEMATOLOGIC AND LYMPHATIC
Ecchymosis
2
1
METABOLIC AND NUTRITIONAL
Weight Loss
3
2
NERVOUS SYSTEM
Dizziness
13
7
Ataxia
6
1
Nystagmus
4
2
Paresthesia
4
1
NEUROPSYCHIATRIC AND COGNITIVE DYSFUNCTION-ALTERED COGNITIVE FUNCTION
Confusion
6
3
Difficulty Concentrating
6
2
Difficulty with Memory
6
2
Mental Slowing
4
2
NEUROPSYCHIATRIC AND COGNITIVE DYSFUNCTION-BEHAVIORAL ABNORMALITIES (NON-PSYCHOSIS-RELATED)
Agitation/Irritability
9
4
Depression
6
3
Insomnia
6
3
Anxiety
3
2
Nervousness
2
1
NEUROPSYCHIATRIC AND COGNITIVE DYSFUNCTION-BEHAVIORAL ABNORMALITIES (PSYCHOSIS-RELATED)
Schizophrenic/Schizophreniform Behavior
2
0
NEUROPSYCHIATRIC AND COGNITIVE DYSFUNCTION-CNS DEPRESSION
Somnolence
17
7
Fatigue
8
6
Tiredness
7
5
NEUROPSYCHIATRIC AND COGNITIVE DYSFUNCTION-SPEECH AND LANGUAGE ABNORMALITIES
Speech Abnormalities
5
2
Difficulties in Verbal Expression
2
<1
RESPIRATORY
Rhinitis
2
1
SKIN AND APPENDAGES
Rash
3
2
SPECIAL SENSES
Diplopia
6
3
Taste Perversion
2
0
Other Adverse Reactions in Clinical Trials: Zonisamide has been administered to 1,598 individuals during all clinical trials, only some of which were placebo-controlled. The frequencies represent the proportion of the 1,598 individuals exposed to zonisamide who experienced an event on at least one occasion. All events are included except those already listed in the previous table or discussed in WARNINGS or PRECAUTIONS, trivial events, those too general to be informative, and those not reasonably associated with zonisamide.
Events are further classified within each category and listed in order of decreasing frequency as follows: frequent occurring in at least 1:100 patients; infrequent occurring in 1:100 to 1:1,000 patients; rare occurring in fewer than 1:1,000 patients.
Body as a Whole: Frequent: Accidental injury, asthenia. Infrequent: Chest pain, flank pain, malaise, allergic reaction, face edema, neck rigidity. Rare: Lupus erythematosus.
Cardiovascular: Infrequent: Palpitation, tachycardia, vascular insufficiency, hypotension, hypertension, thrombophlebitis, syncope, bradycardia. Rare: Atrial fibrillation, heart failure, pulmonary embolus, ventricular extrasystoles.
Digestive: Frequent: Vomiting. Infrequent: Flatulence, gingivitis, gum hyperplasia, gastritis, gastroenteritis, stomatitis, cholelithiasis, glossitis, melena, rectal hemorrhage, ulcerative stomatitis, gastro-duodenal ulcer, dysphagia, gum hemorrhage. Rare: Cholangitis, hematemesis, cholecystitis, cholestatic jaundice, colitis, duodenitis, esophagitis, fecal incontinence, mouth ulceration.
Hematologic and Lymphatic: Infrequent: Leukopenia, anemia, immunodeficiency, lymphadenopathy. Rare: Thrombocytopenia, microcytic anemia, petechia.
Metabolic and Nutritional: Infrequent: Peripheral edema, weight gain, edema, thirst, dehydration. Rare: Hypoglycemia, hyponatremia, lactic dehydrogenase increased, SGOT increased, SGPT increased.
Musculoskeletal: Infrequent: Leg cramps, myalgia, myasthenia, arthralgia, arthritis.
Nervous System: Frequent: Tremor, convulsion, abnormal gait, hyperesthesia, incoordination. Infrequent: Hypertonia, twitching, abnormal dreams, vertigo, libido decreased, neuropathy, hyperkinesia, movement disorder, dysarthria, cerebrovascular accident, hypotonia, peripheral neuritis, reflexes increased. Rare: Dyskinesia, dystonia, encephalopathy, facial paralysis, hypokinesia, hyperesthesia, myoclonus, oculogyric crisis.
Behavioral Abnormalities-Non-Psychosis-Related: Infrequent: Euphoria.
Respiratory: Frequent: Pharyngitis, cough increased. Infrequent: Dyspnea. Rare: Apnea, hemoptysis.
Skin and Appendages: Frequent: Pruritus. Infrequent: Maculopapular rash, acne, alopecia, dry skin, sweating, eczema, urticaria, hirsutism, pustular rash, vesiculobullous rash.
Special Senses: Frequent: Amblyopia, tinnitus. Infrequent: Conjunctivitis, parosmia, deafness, visual field defect, glaucoma. Rare: Photophobia, iritis.
Urogenital: Infrequent: Urinary frequency, dysuria, urinary incontinence, hematuria, impotence, urinary retention, urinary urgency, amenorrhea, polyuria, nocturia. Rare: Albuminuria, enuresis, bladder pain, bladder calculus, gynecomastia, mastitis, menorrhagia.
POST MARKETING EXPERIENCE
The following serious adverse reactions have been reported since approval and use of zonisamide worldwide. These reactions are reported voluntarily from a population of uncertain size; therefore, it is not possible to estimate their frequency or establish a causal relationship to drug exposure.
Acute pancreatitis, rhabdomyolysis, increased creatine phosphokinase, drug reaction with eosinophilia and systemic symptoms (DRESS), acute myopia and secondary angle closure glaucoma, and hyperammonemia and encephalopathy (see WARNINGS).
To report SUSPECTED ADVERSE REACTIONS, contact Sun Pharmaceutical Industries, Inc. at 1-800-818-4555 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Close -
DRUG ABUSE AND DEPENDENCEThe abuse and dependence potential of zonisamide has not been evaluated in human studies (see WARNINGS, Cognitive/Neuropsychiatric Adverse Events subsection). In a series of animal studies ...
The abuse and dependence potential of zonisamide has not been evaluated in human studies (see WARNINGS, Cognitive/Neuropsychiatric Adverse Events subsection). In a series of animal studies, zonisamide did not demonstrate abuse liability and dependence potential. Monkeys did not self-administer zonisamide in a standard reinforcing paradigm. Rats exposed to zonisamide did not exhibit signs of physical dependence of the CNS-depressant type. Rats did not generalize the effects of diazepam to zonisamide in a standard discrimination paradigm after training, suggesting that zonisamide does not have abuse potential of the benzodiazepine-CNS depressant type.
Close -
OVERDOSAGEHuman Experience: Experience with zonisamide daily doses over 800 mg/day is limited. During zonisamide clinical development, three patients ingested unknown amounts of zonisamide as suicide ...
Human Experience: Experience with zonisamide daily doses over 800 mg/day is limited. During zonisamide clinical development, three patients ingested unknown amounts of zonisamide as suicide attempts, and all three were hospitalized with CNS symptoms. One patient became comatose and developed bradycardia, hypotension, and respiratory depression; the zonisamide plasma level was 100.1 mcg/mL measured 31 hours post-ingestion. Zonisamide plasma levels fell with a half-life of 57 hours, and the patient became alert five days later.
Management: No specific antidotes for zonisamide overdosage are available. Following a suspected recent overdose, emesis should be induced or gastric lavage performed with the usual precautions to protect the airway. General supportive care is indicated, including frequent monitoring of vital signs and close observation.
Zonisamide has a long half-life (see CLINICAL PHARMACOLOGY section). Due to the low protein binding of zonisamide (40%), renal dialysis may be effective. The effectiveness of renal dialysis as a treatment of overdose has not been formally studied. A poison control center should be contacted for information on the management of zonisamide overdosage.
Close -
DOSAGE AND ADMINISTRATIONZonisamide capsules are recommended as adjunctive therapy for the treatment of partial seizures in adults. Safety and efficacy in pediatric patients below the age of 16 have not been established ...
Zonisamide capsules are recommended as adjunctive therapy for the treatment of partial seizures in adults. Safety and efficacy in pediatric patients below the age of 16 have not been established. Zonisamide should be administered once or twice daily, using 25 mg, 50 mg or 100 mg capsules. Zonisamide capsules are given orally and can be taken with or without food. Capsules should be swallowed whole.
Adults over Age 16: The prescriber should be aware that, because of the long half-life of zonisamide, up to two weeks may be required to achieve steady state levels upon reaching a stable dose or following dosage adjustment. Although the regimen described below is one that has been shown to be tolerated, the prescriber may wish to prolong the duration of treatment at the lower doses in order to fully assess the effects of zonisamide at steady state, noting that many of the side effects of zonisamide are more frequent at doses of 300 mg per day and above. Although there is some evidence of greater response at doses above 100 to 200 mg/day, the increase appears small and formal dose-response studies have not been conducted.
The initial dose of zonisamide capsules should be 100 mg daily. After two weeks, the dose may be increased to 200 mg/day for at least two weeks. It can be increased to 300 mg/day and 400 mg/day, with the dose stable for at least two weeks to achieve steady state at each level. Evidence from controlled trials suggests that zonisamide doses of 100 to 600 mg/day are effective, but there is no suggestion of increasing response above 400 mg/day (see CLINICAL PHARMACOLOGY, Clinical Studies subsection). There is little experience with doses greater than 600 mg/day.
Patients with Renal or Hepatic Disease: Because zonisamide is metabolized in the liver and excreted by the kidneys, patients with renal or hepatic disease should be treated with caution, and might require slower titration and more frequent monitoring (see CLINICAL PHARMACOLOGY and PRECAUTIONS).
Close -
HOW SUPPLIEDZonisamide capsules are available as 25 mg, 50 mg and 100 mg two-piece hard gelatin capsules. The capsules are printed in black with product code on cap and body “258”, “259” and “260” ...
Zonisamide capsules are available as 25 mg, 50 mg and 100 mg two-piece hard gelatin capsules. The capsules are printed in black with product code on cap and body “258”, “259” and “260”, respectively. Zonisamide capsules are available in bottles of 30, 100, 500 and 1,000 with strengths and colors as follows:
Dosage strength
Capsule color
Pack
NDC#
25 mg
White opaque body with white opaque cap.
Bottle of 30’s with child-resistant cap.
62756-258-01
Bottle of 100’s with child-resistant cap.
62756-258-02
Bottle of 100’s
62756-258-03
Bottle of 1,000’s
62756-258-04
50 mg
White opaque body with light gray opaque cap.
Bottle of 30’s with child-resistant cap.
62756-259-01
Bottle of 100’s with child-resistant cap.
62756-259-02
Bottle of 100’s
62756-259-03
Bottle of 1,000’s
62756-259-04
100 mg
White opaque body with light swedish orange opaque cap.
Bottle of 30’s with child-resistant cap.
62756-260-01
Bottle of 100’s with child-resistant cap.
62756-260-02
Bottle of 100’s
62756-260-03
Bottle of 1,000’s
62756-260-04
Bottle of 500’s
62756-260-05
Store at 25°C (77°F), excursions permitted to 15°-30°C (59°-86°F) [see USP Controlled Room Temperature], in a dry place and protected from light.
Close -
Medication GuideZonisamide (zoe nis’ a mide) Capsules - What is the most important information I should know about Zonisamide Capsules? Zonisamide capsules may cause serious ...
Zonisamide (zoe nis’ a mide) Capsules
What is the most important information I should know about Zonisamide Capsules?
Zonisamide capsules may cause serious side effects, including:- Serious skin rash that can cause death.
- Serious allergic reactions that may affect different parts of the body.
- Less sweating and increase in your body temperature (fever).
- Serious eye problems
- Suicidal thoughts or actions in some people.
- Increased level of acid in your blood (metabolic acidosis).
- Problems with your concentration, attention, memory, thinking, speech, or language.
- Blood cell changes such as reduced red and white blood cell counts.
These serious side effects are described below.
1. Zonisamide capsules may cause a serious skin rash that can cause death. These serious skin reactions are more likely to happen when you begin taking zonisamide capsules within the first 4 months of treatment but may occur at later times.
2. Zonisamide capsules can cause other types of allergic reactions or serious problems that may affect different parts of the body such as your liver, kidneys, heart, or blood cells. You may or may not have a rash with these types of reactions. These reactions can be very serious and can cause death. Call your health care provider right away if you have:
- fever
- severe muscle pain
- rash
- swollen lymph glands
- swelling of your face
- unusual bruising or bleeding
- weakness, fatigue
- yellowing of your skin or the white part of your eyes
3. Zonisamide capsules may cause you to sweat less and to increase your body temperature (fever). You may need to be hospitalized for this. You should watch for decreased sweating and fever, especially when it is hot and especially in children taking zonisamide capsules.
Call your health care provider right away if you have:- high fever, recurring fever, or long lasting fever
- less sweat than normal
4. Zonisamide capsules may cause eye problems. Serious eye problems include:
- sudden decrease in vision with or without eye pain and redness
- a blockage of fluid in the eye causing increased pressure in the eye (secondary angle closure glaucoma)
These eye problems can lead to permanent loss of vision if not treated.
Call your healthcare provider right away if you have any new eye symptoms, including any eye pain or redness or any new problems with your vision.
5. Like other antiepileptic drugs, zonisamide capsules may cause suicidal thoughts or actions in a very small number of people, about 1 in 500.
Call a healthcare provider right away if you have any of these symptoms, especially if they are new, worse, or worry you:
- thoughts about suicide or dying
- attempt to commit suicide
- new or worse depression
- new or worse anxiety
- feeling agitated or restless
- panic attacks
- trouble sleeping (insomnia)
- new or worse irritability
- acting aggressive, being angry, or violent
- acting on dangerous impulses
- an extreme increase in activity and talking (mania)
- other unusual changes in behavior or mood
- Suicidal thoughts or actions can be caused by things other than medicines. If you have suicidal thoughts or actions, your healthcare provider may check for other causes.
How can I watch for early symptoms of suicidal thoughts and actions?
- Pay attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings.
- Keep all follow-up visits with your healthcare provider as scheduled.
Call your healthcare provider between visits as needed, especially if you are worried about symptoms.
Do not stop zonisamide capsules without first talking to a healthcare provider.
Stopping zonisamide capsulessuddenly can cause serious problems. Stopping a seizure medicine suddenly in a patient who has epilepsy can cause seizures that will not stop (status epilepticus).
6. Zonisamide capsules can increase the level of acid in your blood (metabolic acidosis). If left untreated, metabolic acidosis can cause brittle or soft bones (osteoporosis, osteomalacia, osteopenia), kidney stones and can slow the rate of growth in children. Metabolic acidosis can happen with or without symptoms.
Sometimes people with metabolic acidosis will:- feel tired
- not feel hungry (loss of appetite)
- feel changes in heartbeat
- have trouble thinking clearly
Your healthcare provider should do a blood test to measure the level of acid in your blood before and during your treatment with zonisamide capsules.
7. Zonisamide capsules may cause problems with your concentration, attention, memory, thinking, speech, or language.
8. Zonisamide capsules can cause blood cell changes such as reduced red and white blood cell counts. Call your healthcare provider if you develop fever, sore throat, sores in your mouth, or unusual bruising.
Zonisamide capsules can have other serious side effects. For more information ask your healthcare provider or pharmacist. Tell your healthcare provider if you have any side effect that bothers you. Be sure to read the section titled “What are the possible side effects of Zonisamide Capsules?”
What is Zonisamide Capsule?
Zonisamide capsule is a prescription medicine that is used with other medicines to treat partial seizures in adults.
It is not known if zonisamide capsules are safe or effective in children under 16 years of age.
Do not take zonisamide capsules:
Do not take zonisamide capsules if you are allergic to medicines that contain sulfa.
Before taking zonisamide capsules, tell your healthcare provider about all your medical conditions, including if you:- have or have had depression, mood problems or suicidal thoughts or behavior
- have kidney problems
- have liver problems
- have a history of metabolic acidosis (too much acid in your blood)
- have weak, brittle bones or soft bones (osteomalacia, osteopenia or osteoporosis)
- have a growth problem
- are on a diet high in fat called a ketogenic diet
- have diarrhea
- have high blood levels of ammonia
Tell your healthcare provider if you:- are pregnant or plan to become pregnant. Zonisamide capsules may harm your unborn baby. Women who can become pregnant should use effective birth control. Tell your healthcare provider right away if you become pregnant while taking zonisamide capsules.
You and your healthcare provider should decide if you should take zonisamide capsules while you are pregnant.
If you become pregnant while taking zonisamide capsules, talk to your healthcare provider about registering with the North American Antiepileptic Drug Pregnancy Registry. You can enroll in this registry by calling 1-888-233-2334. The purpose of this registry is to collect information about the safety of antiepileptic drugs during pregnancy.
- are breastfeeding or plan to breastfeed. Zonisamide can pass into your breast milk. It is not known if zonisamide in your breast milk can harm your baby. Talk to your healthcare provider about the best way to feed your baby if you take zonisamide capsules.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins or herbal supplements.
How should I take Zonisamide Capsules?- Take zonisamide capsules exactly as prescribed. Your healthcare prescriber may change your dose. Your healthcare provider will tell you how many zonisamide capsules to take.
- Take zonisamide capsules with or without food.
- Swallow the capsules whole.
- If you take too many zonisamide capsules, call your local Poison Control Center or go to the nearest emergency room right away.
- Do not stop taking zonisamide capsules without talking to your healthcare provider. Stopping zonisamide capsules suddenly can cause serious problems, including seizures that will not stop(status epilepticus).
What should I avoid while taking Zonisamide Capsules?- Do not drink alcohol or take other drugs that make you sleepy or dizzy while taking zonisamide capsules until you talk to your health care provider. Zonisamide capsules taken with alcohol or drugs that cause sleepiness or dizziness may make your sleepiness or dizziness worse.
- Do not drive, operate heavy machinery, or do other dangerous activities until you know how zonisamide capsules affect you. Zonisamide capsules can slow your thinking and motor skills.
What are the possible side effects of Zonisamide Capsules?
Zonisamide capsules can cause serious side effects. See “What is the most important information I should know about Zonisamide Capsules?”
Other serious side effects include:
- kidney stones: back pain, stomach pain, or blood in your urine may mean you have kidney stones. Drink plenty of fluids while you take zonisamide capsules to lower your chance of getting kidney stones.
- problems with mood or thinking (new or worse depression; sudden changes in mood, behavior, or loss of contact with reality, sometimes associated with hearing voices or seeing things that are not really there; feeling sleepy or tired; trouble concentrating; speech and language problems). Call your healthcare provider right away if you have any of the symptoms listed above.
- high blood ammonia levels. High ammonia in the blood can affect your mental activities, slow your alertness, make you feel tired, or cause vomiting.
The most common side effects of zonisamide capsules include:- drowsiness
- loss of appetite
- dizziness
- problems with concentration or memory
- trouble with walking and coordination
- agitation or irritability
Side effects can happen at any time, but are more likely to happen during the first several weeks after starting zonisamide capsules.
These are not all of the possible side effects of zonisamide capsules.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store Zonisamide Capsules?- Store zonisamide capsules at 25°C (77°F); excursions permitted to 15°-30°C (59°-86°F)
- Keep zonisamide capsules dry and away from light
Close
Keep zonisamide capsules and all medicines out of the reach of children.
General Information about the safe and effective use of Zonisamide Capsules
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use zonisamide capsules for a condition for which it was not prescribed. Do not give zonisamide capsules to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about zonisamide capsules that is written for health professionals.
What are the ingredients in Zonisamide Capsules?
Active ingredient: zonisamide
Inactive ingredients: microcrystalline cellulose, hydrogenated vegetable oil, gelatin, and titanium dioxide.
In addition, individual empty hard gelatin capsule shell contains:
50 mg: Black iron oxide
100 mg: FD&C Blue # 1 and FD&C Red # 40.
The imprinting ink contains black iron oxide, shellac glaze, propylene glycol and also contains either FD & C Blue No. 2, FD & C Red No. 40, FD & C Blue No. 1 and D & C Yellow No.10 or strong ammonia solution and potassium hydroxide.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Distributed by:
Sun Pharmaceutical Industries, Inc.
Cranbury, NJ 08512
Manufactured by:
Sun Pharmaceutical Ind. Ltd.
Halol-Baroda Highway,
Halol-389 350, Gujarat, India.
PJPI 0192G
ISS 06/2020 -
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL-50mgNDC 62756-259-01 - Zonisamide Capsules - 50 mg - Rx only - 30 Capsules - SUN PHARMA - PHARMACIST: Please dispense with Medication Guide provided separately to each patient.
NDC 62756-259-01
Close
Zonisamide Capsules
50 mg
Rx only
30 Capsules
SUN PHARMA
PHARMACIST: Please dispense with Medication Guide provided separately to each patient.

-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL-25mgNDC 62756-258-01 - Zonisamide Capsules - 25 mg - Rx only - 30 Capsules - SUN PHARMA - PHARMACIST: Please dispense with Medication Guide provided separately to each patient.
NDC 62756-258-01
Close
Zonisamide Capsules
25 mg
Rx only
30 Capsules
SUN PHARMA
PHARMACIST: Please dispense with Medication Guide provided separately to each patient.

-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL-100mgNDC 62756-260-01 - Zonisamide Capsules - 100 mg - Rx only - 30 Capsules - SUN PHARMA - PHARMACIST: Please dispense with Medication Guide provided separately to each patient.
NDC 62756-260-01
Close
Zonisamide Capsules
100 mg
Rx only
30 Capsules
SUN PHARMA
PHARMACIST: Please dispense with Medication Guide provided separately to each patient.

-
INGREDIENTS AND APPEARANCEProduct Information
ZONISAMIDE zonisamide capsule Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:62756-258 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZONISAMIDE (UNII: 459384H98V) (ZONISAMIDE - UNII:459384H98V) ZONISAMIDE 25 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERROSOFERRIC OXIDE (UNII: XM0M87F357) SHELLAC (UNII: 46N107B71O) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) AMMONIA (UNII: 5138Q19F1X) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) Product Characteristics Color WHITE (white opaque) , WHITE (white opaque) Score no score Shape CAPSULE Size 15mm Flavor Imprint Code 258;258 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62756-258-01 30 in 1 BOTTLE; Type 0: Not a Combination Product 03/17/2006 2 NDC:62756-258-02 100 in 1 BOTTLE; Type 0: Not a Combination Product 03/17/2006 3 NDC:62756-258-03 100 in 1 BOTTLE; Type 0: Not a Combination Product 03/17/2006 4 NDC:62756-258-04 1000 in 1 BOTTLE; Type 0: Not a Combination Product 03/17/2006 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA077634 03/17/2006 ZONISAMIDE zonisamide capsule Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:62756-259 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZONISAMIDE (UNII: 459384H98V) (ZONISAMIDE - UNII:459384H98V) ZONISAMIDE 50 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERROSOFERRIC OXIDE (UNII: XM0M87F357) SHELLAC (UNII: 46N107B71O) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) AMMONIA (UNII: 5138Q19F1X) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) Product Characteristics Color WHITE (white opaque) , GRAY (light gray opaque) Score no score Shape CAPSULE Size 16mm Flavor Imprint Code 259;259 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62756-259-01 30 in 1 BOTTLE; Type 0: Not a Combination Product 03/17/2006 2 NDC:62756-259-02 100 in 1 BOTTLE; Type 0: Not a Combination Product 03/17/2006 3 NDC:62756-259-03 100 in 1 BOTTLE; Type 0: Not a Combination Product 03/17/2006 4 NDC:62756-259-04 1000 in 1 BOTTLE; Type 0: Not a Combination Product 03/17/2006 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA077634 03/17/2006 ZONISAMIDE zonisamide capsule Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:62756-260 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZONISAMIDE (UNII: 459384H98V) (ZONISAMIDE - UNII:459384H98V) ZONISAMIDE 100 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERROSOFERRIC OXIDE (UNII: XM0M87F357) SHELLAC (UNII: 46N107B71O) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) AMMONIA (UNII: 5138Q19F1X) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) Product Characteristics Color WHITE (white opaque) , ORANGE (swedish orange opaque) Score no score Shape CAPSULE Size 20mm Flavor Imprint Code 260;260 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62756-260-01 30 in 1 BOTTLE; Type 0: Not a Combination Product 03/17/2006 2 NDC:62756-260-02 100 in 1 BOTTLE; Type 0: Not a Combination Product 03/17/2006 3 NDC:62756-260-03 100 in 1 BOTTLE; Type 0: Not a Combination Product 03/17/2006 4 NDC:62756-260-04 1000 in 1 BOTTLE; Type 0: Not a Combination Product 03/17/2006 5 NDC:62756-260-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 03/17/2006 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA077634 03/17/2006 Labeler - Sun Pharmaceutical Industries, Inc. (146974886)
CloseEstablishment Name Address ID/FEI Business Operations Sun Pharmaceutical Industries Limited 725959238 ANALYSIS(62756-258, 62756-259, 62756-260) , MANUFACTURE(62756-258, 62756-259, 62756-260)
Find additional resources
(also available in the left menu)Safety
Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
RxNorm
ZONISAMIDE capsule
Get Label RSS Feed for this Drug
ZONISAMIDE capsule
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=3164d438-90bf-420a-9a56-5498b987f91c
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
ZONISAMIDE capsule
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 62756-258-01 |
| 2 | 62756-258-02 |
| 3 | 62756-258-03 |
| 4 | 62756-258-04 |
| 5 | 62756-259-01 |
| 6 | 62756-259-02 |
| 7 | 62756-259-03 |
| 8 | 62756-259-04 |
| 9 | 62756-260-01 |
| 10 | 62756-260-02 |
| 11 | 62756-260-03 |
| 12 | 62756-260-04 |
| 13 | 62756-260-05 |



